
Metal acetylacetonates
Encyclopedia
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion
and metal ions, usually transition metal
s. The ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl. Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis
, and precursors to industrial hydroformylation
catalysts. C5H7O2− in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).
Addition of base assists the removal of a proton from acetylacetone and shifts the equilibrium in favour of the complex. Both oxygen centres bind to the metal to form a six-membered chelate ring. In some cases the chelate effect is so strong that no added base is needed to form the complex. Some complexes are prepared by metathesis using Tlacac.
:
This reaction requires no base. The complex TiCl2(acac)2 is fluxional in solution, the NMR spectrum exhibiting a single methyl resonance at room temperature.
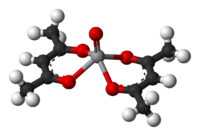 Vanadyl acetylacetonate
Vanadyl acetylacetonate
is a blue complex with the formula V(O)(acac)2. This complex features the vanadyl group, and many related compounds are known. The molecule is square pyramidal, with idealized C2v symmetry. The complex catalyzes epoxidation of allylic alcohols by peroxides.
is a typical octahedral complex containing three acac- ligands. Like most such compounds, it is highly soluble in nonpolar organic solvents. This particular complex, which has a three unpaired electrons, is used as a spin relaxation agent to improve the sensitivity in quantitative Carbon-13 NMR
spectroscopy.
manganese(iii)-3d-balls.png) It is prepared by the direct reaction of acetylacetone and potassium permanganate
It is prepared by the direct reaction of acetylacetone and potassium permanganate
. In terms of electronic structure, Mn(acac)3 is high spin. Its distorted octahedral structure reflects geometric distortions due to the Jahn-Teller effect
. The two most common structures for this complex include one with tetrahedral elongation and one with tetragonal compression. For the elongation, two Mn-O bonds are 2.12 Å while the other four are 1.93 Å. For the compression, two Mn-O bonds are 1.95 and the other four are 2.00 Å. The effects of the tetrahedral elongation are noticeably more significant than the effects of the tetragonal compression.
Mn(acac)3, a one-electron oxidant, is used for coupling phenols.
complex that is highly soluble in organic solvents. It is configurationationally labile, high-spin complex with five unpaired electrons. It is occasionally used as a catalyst precursor. Although configurationationally labile, Fe(acac)3 has been partially resolved. The ferrous complex Fe(acac)2 is oligomeric.
(has a non-superimposable mirror image). Many such complexes have been resolved, but the premier example is Co(acac)3.
The complex Co(acac)2, like the related nickel complex, exists octahedral complexes with two additional ligands. The anhydrous form exists as the tetramer [Co(acac)2]4. Like the trimeric nickel complex, this tetramer shows ferromagnetic interactions at low temperatures.
nickel(ii)-3d-sticks.png) Nickel(II) complexes are generally 6-coordinate, octahedral. Monomeric Ni(acac)2 is therefore coordinatively unsaturated and behaves as a Lewis acid
Nickel(II) complexes are generally 6-coordinate, octahedral. Monomeric Ni(acac)2 is therefore coordinatively unsaturated and behaves as a Lewis acid
. Water can serve as a Lewis base to give the octahedral adduct
[Ni(acac)2(H2O)2]. Dehydration of this complex causes trimerization to tive [Ni(acac)2]3 to be , in which some oxygen centres bridge
two nickel ions. This complex is a benzene-soluble, emerald green solid, which is widely employed in the preparation of Ni(O) complexes, e.g. bis(cyclooctadiene)nickel(0)
. Upon exposure to the atmosphere, [Ni(acac)2]3 converts back to the chalky green monomeric dihydrate. Bulky beta-diketonates give red, monomeric, square-planar complexes.
[Ni(acac)2]3 has interesting magnetic properties
. Down to about 80K it exhibits normal paramagnetism
with an effective magnetic moment of 3.2 μB, close to the spin-only moment expected of a d8 ion with two unpaired electrons. The effective moment rises to 4.1μB at 4.3K, due to ferromagnetic exchange interaction
s involving all three nickel ions.
_acac.png) Cu(acac)2 is prepared by treating acetylacetone with aqueous Cu(NH3)42+. It is available commercially, catalyzes coupling and carbene transfer reactions.
Cu(acac)2 is prepared by treating acetylacetone with aqueous Cu(NH3)42+. It is available commercially, catalyzes coupling and carbene transfer reactions.
Unlike the copper(II) derivative, copper(I) acetylacetonate is an air sensitive
oligomeric species. It is employed to catalyze Michael additions.
. Dehydration of this species gives the hygroscopic anhydrous derivative (m.p. 127 °C). This more volatile derivative has been used as a precursor to films of ZnO
.
s often adopt coordination numbers >8. In such cases, derivatives of acac- are more common. One example is the NMR shift reagent Eufod
, Eu(OCC(CH3)3CHCOC3F7)3. This complex is a Lewis acid and forming adducts with a variety of hard bases.
) contain one carbon-bonded acac ligand. The IR spectra of O-bonded acetylacetonates are characterized by relatively low-energy νCO bands of 1535 cm−1, whereas in carbon-bonded acetylacetonates, the carbonyl vibration occurs closer to the normal range for ketonic C=O, i.e. 1655 cm−1.
Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
and metal ions, usually transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s. The ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl. Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, and precursors to industrial hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
catalysts. C5H7O2− in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).
Synthesis
A general method of synthesis is to treat a metal salt with acetylacetone, acacH:- Mz+ + z (acacH) M(acac)z + z H+
Addition of base assists the removal of a proton from acetylacetone and shifts the equilibrium in favour of the complex. Both oxygen centres bind to the metal to form a six-membered chelate ring. In some cases the chelate effect is so strong that no added base is needed to form the complex. Some complexes are prepared by metathesis using Tlacac.
Titanium bis(acetylacetonate)dichloride
Treatment of TiCl4 with acetylacetone gives cis-TiCl2(acac)2, a red-coloured, octahedral complex with C2-symmetrySymmetry group
The symmetry group of an object is the group of all isometries under which it is invariant with composition as the operation...
:
- TiCl4 + 2 Hacac → TiCl2(acac)2 + 2 HCl
This reaction requires no base. The complex TiCl2(acac)2 is fluxional in solution, the NMR spectrum exhibiting a single methyl resonance at room temperature.
Vanadyl acetylacetonate

Vanadyl acetylacetonate
Vanadyl acetylacetonate is the chemical compound with the formula VO2. This blue-green coordination complex consists of the vanadyl group, VO2+, bound to two acetylacetonate anions, acac−. Like other charge-neutral acetylacetonates, this complex is soluble in organic solvents.-Synthesis:The...
is a blue complex with the formula V(O)(acac)2. This complex features the vanadyl group, and many related compounds are known. The molecule is square pyramidal, with idealized C2v symmetry. The complex catalyzes epoxidation of allylic alcohols by peroxides.
Chromium(III) acetylacetonate
Cr(acac)3Chromium(III) acetylacetonate
Chromium acetylacetonate is the coordination compound with the formula Cr3, sometimes designated as Cr3. This purplish coordination complex is used in NMR spectroscopy as a relaxation agent because of its solubility in nonpolar organic solvents and its paramagnetism.-Synthesis and structure:The...
is a typical octahedral complex containing three acac- ligands. Like most such compounds, it is highly soluble in nonpolar organic solvents. This particular complex, which has a three unpaired electrons, is used as a spin relaxation agent to improve the sensitivity in quantitative Carbon-13 NMR
Carbon-13 NMR
Carbon-13 NMR is the application of nuclear magnetic resonance spectroscopy to carbon. It is analogous to proton NMR and allows the identification of carbon atoms in an organic molecule just as proton NMR identifies hydrogen atoms...
spectroscopy.
Manganese(III) acetylacetonate
manganese(iii)-3d-balls.png)
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
. In terms of electronic structure, Mn(acac)3 is high spin. Its distorted octahedral structure reflects geometric distortions due to the Jahn-Teller effect
Jahn-Teller effect
The Jahn–Teller effect, sometimes also known as Jahn–Teller distortion, or the Jahn–Teller theorem, describes the geometrical distortion of non-linear molecules under certain situations. This electronic effect is named after Hermann Arthur Jahn and Edward Teller, who proved, using group theory,...
. The two most common structures for this complex include one with tetrahedral elongation and one with tetragonal compression. For the elongation, two Mn-O bonds are 2.12 Å while the other four are 1.93 Å. For the compression, two Mn-O bonds are 1.95 and the other four are 2.00 Å. The effects of the tetrahedral elongation are noticeably more significant than the effects of the tetragonal compression.
Mn(acac)3, a one-electron oxidant, is used for coupling phenols.
Iron acetylacetonates
Ferric acetylacetonate, Fe(acac)3, is a red high-spinMagnetochemistry
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or...
complex that is highly soluble in organic solvents. It is configurationationally labile, high-spin complex with five unpaired electrons. It is occasionally used as a catalyst precursor. Although configurationationally labile, Fe(acac)3 has been partially resolved. The ferrous complex Fe(acac)2 is oligomeric.
Ruthenium acetylacetonates
Like iron, Ru forms a very stable tris(acetylacetonate). Reduction of this Ru(III) derivative in the presence of other ligands affords mixed ligand complexes, e.g. Ru(acac)2(alkene)2.Cobalt acetylacetonates
Co(acac)3 is low-spin, diamagnetic complex. Like other compounds of the type M(acac)3, this complex is chiralChirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
(has a non-superimposable mirror image). Many such complexes have been resolved, but the premier example is Co(acac)3.
The complex Co(acac)2, like the related nickel complex, exists octahedral complexes with two additional ligands. The anhydrous form exists as the tetramer [Co(acac)2]4. Like the trimeric nickel complex, this tetramer shows ferromagnetic interactions at low temperatures.
Iridium acetylacetonates
Ir(acac)3 is the formula for two isomers, trans-Ir(acac)2(CH(COMe)2)(H2O) and the more conventional D3-symmetric Ir(acac)3. The C-bonded derivative is a precursor to homogeneous catalysts for C-H activation and related chemistries. Iridium(I) derivatives include square-planar Ir(acac)(CO)2 (C2v-symmetry).Nickel(II) acetylacetonate
nickel(ii)-3d-sticks.png)
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
. Water can serve as a Lewis base to give the octahedral adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
[Ni(acac)2(H2O)2]. Dehydration of this complex causes trimerization to tive [Ni(acac)2]3 to be , in which some oxygen centres bridge
Bridging ligand
A bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are...
two nickel ions. This complex is a benzene-soluble, emerald green solid, which is widely employed in the preparation of Ni(O) complexes, e.g. bis(cyclooctadiene)nickel(0)
Bis(cyclooctadiene)nickel(0)
Bisnickel is the organometallic compound with the formula Ni2. This air-sensitive yellow solid is a common source of Ni in chemical synthesis....
. Upon exposure to the atmosphere, [Ni(acac)2]3 converts back to the chalky green monomeric dihydrate. Bulky beta-diketonates give red, monomeric, square-planar complexes.
[Ni(acac)2]3 has interesting magnetic properties
Magnetochemistry
Magnetochemistry is concerned with the magnetic properties of chemical compounds. Magnetic properties arise from the spin and orbital angular momentum of the electrons contained in a compound. Compounds are diamagnetic when they contain no unpaired electrons. Molecular compounds that contain one or...
. Down to about 80K it exhibits normal paramagnetism
Paramagnetism
Paramagnetism is a form of magnetism whereby the paramagnetic material is only attracted when in the presence of an externally applied magnetic field. In contrast with this, diamagnetic materials are repulsive when placed in a magnetic field...
with an effective magnetic moment of 3.2 μB, close to the spin-only moment expected of a d8 ion with two unpaired electrons. The effective moment rises to 4.1μB at 4.3K, due to ferromagnetic exchange interaction
Exchange interaction
In physics, the exchange interaction is a quantum mechanical effect without classical analog which increases or decreases the expectation value of the energy or distance between two or more identical particles when their wave functions overlap...
s involving all three nickel ions.
Copper acetylacetonate
_acac.png)
Unlike the copper(II) derivative, copper(I) acetylacetonate is an air sensitive
Air-free technique
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen...
oligomeric species. It is employed to catalyze Michael additions.
Zinc acetylacetonate
The monoaquo complex Zn(acac)2H2O (m.p. 138-140 °C) is pentacoordinate, adopting a square pyramidal structure. The complex is of some use in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. Dehydration of this species gives the hygroscopic anhydrous derivative (m.p. 127 °C). This more volatile derivative has been used as a precursor to films of ZnO
Zinc oxide
Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder that is insoluble in water. The powder is widely used as an additive into numerous materials and products including plastics, ceramics, glass, cement, rubber , lubricants, paints, ointments, adhesives, sealants,...
.
Acetylacetonates of the other elements
Colourless, diamagnetic Al(acac)3 is structurally similar to other tris complexes, e.g. [Fe(acac)3]. The trisacetylacetonates of the lanthanideLanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s often adopt coordination numbers >8. In such cases, derivatives of acac- are more common. One example is the NMR shift reagent Eufod
Eufod
EuFOD is the chemical compound with the formula Eu3, also called Eu3. This coordination compound is used primarily as a shift reagent in NMR spectroscopy...
, Eu(OCC(CH3)3CHCOC3F7)3. This complex is a Lewis acid and forming adducts with a variety of hard bases.
C-bonded acetylacetonates
C5H7O2− in some cases also binds to metals through the central carbon atom (C3); this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III). The complexes Ir(acac)3 and corresponding Lewis-base adducts Ir(acac)3L (L = an amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
) contain one carbon-bonded acac ligand. The IR spectra of O-bonded acetylacetonates are characterized by relatively low-energy νCO bands of 1535 cm−1, whereas in carbon-bonded acetylacetonates, the carbonyl vibration occurs closer to the normal range for ketonic C=O, i.e. 1655 cm−1.


_acac.png)