
Lithium burning
Encyclopedia
Lithium
is generally present in brown dwarf
s and not in low-mass stars. Stars, which achieve the high temperature (2.5 × 106 K) necessary for fusing hydrogen
, rapidly deplete their lithium. This occurs by a collision of lithium-7 and a proton
producing two helium-4
nuclei. The temperature necessary for this reaction is just below the temperature necessary for hydrogen fusion. Convection in low-mass stars ensures that lithium in the whole volume of the star is depleted. Therefore, the presence of the lithium line
in a candidate brown dwarf's spectrum is a strong indicator that it is indeed substellar.
From a study of lithium abundances in 53 T Tauri star
s, it has been found that lithium depletion varies strongly with size, suggesting that lithium burning by the P-P chain
, during the last highly convective and unstable stages during the pre–main sequence later phase of the Hayashi contraction
may be one of the main sources of energy for T Tauri stars. Rapid rotation tends to improve mixing and increase the transport of lithium into deeper layers where it is destroyed. T Tauri stars generally increase their rotation rates as they age, through contraction and spin-up, as they conserve angular momentum. This causes an increased rate of lithium loss with age. Lithium burning will also increase with higher temperatures and mass, and will last for at most a little over 100 million years.
The P-P chain for lithium burning is as follows
|- style="height:2em;"
| ||+ || ||→ |||| ||(unstable)
|- style="height:2em;"
| ||+ || ||→ || ||+
|- style="height:2em;"
| ||+ || ||→ || || ||(unstable)
|- style="height:2em;"
| || || ||→ ||2 ||+ energy
|}
It will not occur in stars less than sixty times the mass of Jupiter. In this way, the rate of lithium depletion can be used to calculate the age of the star.
The use of lithium to distinguish candidate brown dwarfs from low-mass stars is commonly referred to as the lithium test, and was pioneered by Rafael Rebolo and colleagues. Heavier stars like our sun can retain lithium in their outer atmospheres, which never get hot enough for lithium depletion, but those are distinguishable from brown dwarfs by their size. Brown dwarfs at the high end of their mass range can be hot enough to deplete their lithium when they are young. Dwarfs of mass greater than 65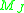 can burn off their lithium by the time they are half a billion years old[Kulkarni], thus this test is not perfect.
can burn off their lithium by the time they are half a billion years old[Kulkarni], thus this test is not perfect.
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
is generally present in brown dwarf
Brown dwarf
Brown dwarfs are sub-stellar objects which are too low in mass to sustain hydrogen-1 fusion reactions in their cores, which is characteristic of stars on the main sequence. Brown dwarfs have fully convective surfaces and interiors, with no chemical differentiation by depth...
s and not in low-mass stars. Stars, which achieve the high temperature (2.5 × 106 K) necessary for fusing hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, rapidly deplete their lithium. This occurs by a collision of lithium-7 and a proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
producing two helium-4
Helium-4
Helium-4 is a non-radioactive isotope of helium. It is by far the most abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on earth. Its nucleus is the same as an alpha particle, consisting of two protons and two neutrons. Alpha decay of heavy...
nuclei. The temperature necessary for this reaction is just below the temperature necessary for hydrogen fusion. Convection in low-mass stars ensures that lithium in the whole volume of the star is depleted. Therefore, the presence of the lithium line
Spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies.- Types of line spectra :...
in a candidate brown dwarf's spectrum is a strong indicator that it is indeed substellar.
From a study of lithium abundances in 53 T Tauri star
T Tauri star
T Tauri stars are a class of variable stars named after their prototype – T Tauri. They are found near molecular clouds and identified by their optical variability and strong chromospheric lines.-Characteristics:...
s, it has been found that lithium depletion varies strongly with size, suggesting that lithium burning by the P-P chain
Proton-proton chain reaction
The proton–proton chain reaction is one of several fusion reactions by which stars convert hydrogen to helium, the primary alternative being the CNO cycle. The proton–proton chain dominates in stars the size of the Sun or smaller....
, during the last highly convective and unstable stages during the pre–main sequence later phase of the Hayashi contraction
Hayashi track
The Hayashi track is a path taken by protostars in the Hertzsprung–Russell diagram after the protostellar cloud has reached approximate hydrostatic equilibrium...
may be one of the main sources of energy for T Tauri stars. Rapid rotation tends to improve mixing and increase the transport of lithium into deeper layers where it is destroyed. T Tauri stars generally increase their rotation rates as they age, through contraction and spin-up, as they conserve angular momentum. This causes an increased rate of lithium loss with age. Lithium burning will also increase with higher temperatures and mass, and will last for at most a little over 100 million years.
The P-P chain for lithium burning is as follows
- {| border="0"
|- style="height:2em;"
| ||+ || ||→ |||| ||(unstable)
|- style="height:2em;"
| ||+ || ||→ || ||+
|- style="height:2em;"
| ||+ || ||→ || || ||(unstable)
|- style="height:2em;"
| || || ||→ ||2 ||+ energy
|}
It will not occur in stars less than sixty times the mass of Jupiter. In this way, the rate of lithium depletion can be used to calculate the age of the star.
The use of lithium to distinguish candidate brown dwarfs from low-mass stars is commonly referred to as the lithium test, and was pioneered by Rafael Rebolo and colleagues. Heavier stars like our sun can retain lithium in their outer atmospheres, which never get hot enough for lithium depletion, but those are distinguishable from brown dwarfs by their size. Brown dwarfs at the high end of their mass range can be hot enough to deplete their lithium when they are young. Dwarfs of mass greater than 65
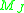 can burn off their lithium by the time they are half a billion years old[Kulkarni], thus this test is not perfect.
can burn off their lithium by the time they are half a billion years old[Kulkarni], thus this test is not perfect.

