Isotopic labeling
Encyclopedia
Isotopic labeling is a technique for tracking the passage of a sample of substance through a system. The substance is 'labeled' by including unusual isotope
s in its chemical composition. If these unusual isotopes are later detected in a certain part of the system, they must have come from the labeled substance.
In ordinary isotopic labeling, there are two ways to detect the presence of labeling isotopes. Since isotopes have different mass
es, they can be separated using mass spectrometry
. Another consequence of the difference in mass is that molecules containing isotopes have different vibrational modes; these can be detected by infrared spectroscopy
.
Isotopic labeling can also be used to study chemical reaction
s. In this method specific atoms are replaced by an isotope in a reactant molecule which then participates in a chemical reaction. With spectroscopy
, nuclear magnetic resonance
spectroscopy for example, it is now possible to identify where a particular molecular fragment in the reactant ends up as a particular fragment in one of the reaction products.
An example of the use of isotopic labeling is the study of phenol
(C6H5OH) in water by replacing common hydrogen
(protium) with deuterium
(deuterium labeling). Upon adding phenol to deuterated water
(water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.
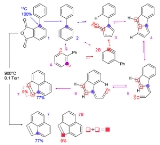
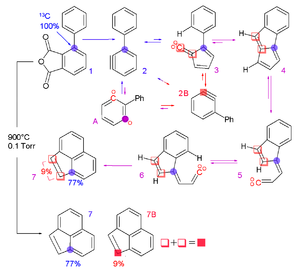
(F6P) which has 6 carbon atoms with a label 13C at carbon position 1 and 2. F6P becomes T3P in which the labeled 1,2-13C F6P produces a 2,3-13C T3P. The 2,3-13C T3P can now introduce a different labeled F6P, a 5,6-13C F6P. The figure shows the use of stable isotope labeling to discover the carbon rearrangement through reactions using position specific labeled compounds.
(MFA) using stable isotope labeling is an important tool to figuring out the metabolic pathways and reactions that occur within a cell. An isotopic label is fed to the cell, then the cell is allowed to grow, and finally the isotope pattern of the output metabolite is determined. The output isotope pattern provides valuable information which can be used to find the amount of flux
, rate of conversion from reactants to products, through each reaction.
Figure 1 demonstrates the ability to use different labels to determine the flux through a certain reaction. Let us assume, the original metabolite
, a three carbon metabolite, has the ability to split into a two carbon and one carbon metabolite, then recombine or remain a three carbon metabolite. If the reaction is provided with two isotopes of the metabolite in equal proportion, one completely labeled, commonly known as uniformly labeled, and one unlabeled. The pathway down the left side of the diagram does not display any change in the metabolites, while the right side shows the split and recombination. As shown, if the metabolite only takes the pathway down the left side, it remains in a 50-50 ratio of uniformly labeled to unlabeled metabolite. If the metabolite only takes the right side new labeling patterns can occur, all in equal proportion. Other proportions can occur depending on how much of the original metabolite follows the left side of the pathway versus the right side of the pathway. Here the proportions are shown for a situation in which half of the metabolites take the left side and half the right, but other proportions can occur. By measuring the proportion of the differently labeled metabolites, the flux through each reaction can be determined.
MFA results in a flux map which shows the amount of reactants being converted to products for each reaction. In most flux maps, the thicker the arrow, the larger the flux value of the reaction.
Figure 2 is an example of the MFA of the conversion of Glucose to Pyruvate. This is an example of a more complex flux map in comparison to Figure 1. In this map the tracking of the isotope is useful in figuring out what genes are needed to be added to maximize the production of a specific product.
Proton NMR was the first technique that was used for 13C-labeling experiments. Using this method, each single protonated carbon position inside a particular metabolite pool can be observed separately from the other positions.5 This allows the percentage of isotopomers labeled at that specific position to be known. The limit to proton NMR was that if there are n carbon atoms in a metabolite, there can only be at most n different positional enrichment values, which is only a small fraction of the total isotopomer information. Although the use of proton NMR labeling is limiting, pure proton NMR experiments are much easier to evaluate than experiments with more isotopomer information.
13C NMR spectrum allows a more detailed isotopomer distribution because a labeled carbon produces different hyperfine splitting signals depending on the labeling state of its direct neighbors in the molecule. 6. A singlet peak emerges if the neighboring carbons are not labeled. A doublet peak emerges if only one neighboring carbon is labeling. The size of the doublet split depends on the functional group of the neighboring carbon. If two neighboring carbons are labeled, a doublet of doublets may degenerate into a triplet if the doublet splittings are equal.
The drawbacks to using NMR techniques for Metabolic Flux Analysis purposes is that it is a rather specialized discipline which is different from other NMR applictions. An NMR spectrometer may not be directly available for all research teams. The optimization iof NMR measurement parameters and proper analysis of peak structures requires a skilled NMR specialist. Certain metabolites may require specialized measurement procedures to obtain additional isotopomer data.
In addition, specially adapted software tools are needed to determine the precie quantity of peak areas as well as identifying the decomposition of entangled singlet, doublet, and triplet peaks.
Another method as opposed to NMR is mass spectrometry which is more applicable and sensitive to MFA experiments. All MS instruments work directly with hydrolysate.
In GC-MS, the MS is couple to a gas chromatograph to separate the compounds of the hydrolysate.
The compounds eluting from the GC column are then ionized and simultaneously fragmented. The benefit in using GC-MS is that not only the mass isotopomers of the molecular ion are measured but also the mass isotopomer spectrum of several fragments, which significantly increases the measured information.
MS instruments divide a particular isotopomer distribution by its molecular weight, meaning that all isotopomers of a particular metabolite contains the same number of labeled carbon atoms are collected in one peak signal. Because every isotopomer contributes to exactly one peak in the MS spectrum, A percentage value can then be calculated for each peak, yielding the mass isotopomer fraction. 5
The drawbacks to using MS techniques is that the sample must be prepared by chemical derivation in order to obtain molecules with charge. In addition, the retention time of differently labeled isotopomers in the GC column also depends on the isotopomer. The natural abundance of other atoms than carbon also leads to a disturbance in the mass isotopomer spectrum.
s in its chemical composition. When these decay
, their presence can be determined by detecting the radiation
emitted by them. Radioisotopic labeling is a special case of isotopic labeling.
For these purposes, a particularly useful type of radioactive decay is positron emission
. When a positron collides with an electron, it releases two high-energy photon
s traveling in diametrically opposite directions. If the positron is produced within a solid object, it is likely to do this before traveling more than a millimeter. If both of these photons can be detected, the location of the decay event can be determined very precisely.
Strictly speaking, radioisotopic labeling includes only cases where radioactivity is artificially introduced by experimenters, but some natural phenomena allow similar analysis to be performed. In particular, radiometric dating
uses a closely related principle.
 An isotopic tracer, (also "isotopic marker" or "isotopic label"), is used in chemistry
An isotopic tracer, (also "isotopic marker" or "isotopic label"), is used in chemistry
and biochemistry
to help understand chemical reactions
and interactions. In this technique, one or more of the atom
s of the molecule
of interest is substituted for an atom of the same chemical element
, but of a different (often radioactive, such as in radioactive tracing
) isotope
. Because the atom has the same number of protons, it will behave in almost exactly the same way chemically as other atoms in the compound, and with few exceptions will not interfere with the reaction under investigation. The difference in the number of neutron
s, however, means that it can be detected separately from the other atoms of the same element.
NMR
typically uses this type of technique to investigate the mechanisms of chemical reactions (basically trying to find out which starting atom ends up where after a reaction), because NMR detects not only isotopic differences, but also gives an indication of the position of the atom.
Mass spectrometry can also be used with this technique, since mass spectra recorded with sufficiently high resolution can distinguish among isotopes based on the different masses resulting from the different number of neutrons.
Autoradiograph
s of gels in gel electrophoresis
can also take advantage of this approach. In this technique, radioactive isotopes are used. The radiation emitted by compounds containing the radioactive isotopes darkens a piece of photographic film
, recording the position of these compounds relative to one another in the gel.
Isotopic (isotope) tracers are some of the most important tools in geology
, because they can be used to understand complex mixing process in earth systems. Isotope tracers are almost always used in the form of isotope ratios. By studying the ratio between two isotopes of the same element, we avoid effects involving the overall abundance of the element, which would usually swamp the much smaller variations in isotopic abundances. Further discussion of the application of isotopic tracers in geology is covered under the heading of isotope geochemistry
.
Isotopic tracers are usually subdivided into two categories: radiogenic isotope tracers and stable isotope
tracers. A radiogenic isotope tracer involves an isotope produced by radioactive decay
, which is usually ratioed against a non-radiogenic isotope (whose abundance in the earth does not vary due to radioactive decay).
A stable isotope tracer involves only non-radiogenic isotopes. In this case, relative variations in abundance between the two isotopes is most often caused by mass-dependent fractionation. In theory, any element with two stable isotopes can be used as an isotopic tracer. However, the most commonly used stable isotope tracers involve relatively light isotopes which readily undergo fractionation in natural systems. See also isotopic signature
.
Various isotopes of lead can be used to study circulation on a global scale. Different oceans (i.e. the Atlantic, Pacific, Indian, etc.) have different isotopic signatures. This results from differences in isotopic ratios of sediments and rocks within the different oceans. Because the different isotopes of lead have half-lives of 50-200 years, there is not enough time for the isotopic ratios to be homogenized throughout the whole ocean. Therefore, precise analysis of Pb isotopic ratios can be used to study the circulation of the different oceans.
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s in its chemical composition. If these unusual isotopes are later detected in a certain part of the system, they must have come from the labeled substance.
In ordinary isotopic labeling, there are two ways to detect the presence of labeling isotopes. Since isotopes have different mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
es, they can be separated using mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
. Another consequence of the difference in mass is that molecules containing isotopes have different vibrational modes; these can be detected by infrared spectroscopy
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
.
Isotopic labeling can also be used to study chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s. In this method specific atoms are replaced by an isotope in a reactant molecule which then participates in a chemical reaction. With spectroscopy
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
, nuclear magnetic resonance
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
spectroscopy for example, it is now possible to identify where a particular molecular fragment in the reactant ends up as a particular fragment in one of the reaction products.
An example of the use of isotopic labeling is the study of phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
(C6H5OH) in water by replacing common hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(protium) with deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
(deuterium labeling). Upon adding phenol to deuterated water
Heavy water
Heavy water is water highly enriched in the hydrogen isotope deuterium; e.g., heavy water used in CANDU reactors is 99.75% enriched by hydrogen atom-fraction...
(water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.
Stable isotope labeling
Stable Isotope Labeling involves the use of non-radioactive isotopes that can act as a tracer in several biological systems. Some of the most common stable isotopes are 2H, 13C, and 15N, which can further be produced into NMR solvents, amino acids, nucleic acids, lipids, common metabolites and cell growth media5. The compounds produced using stable isotope labels can either be specified by the percentage of labeled isotopes i.e. 30% uniformly labeled 13C glucose which would contain three 13C atoms per seven 13C atoms or specifically labeled at certain positions on the compound i.e. 1-13C glucose which is labeled at carbon position 1 of glucose. Figure 2 shows a network of reactions adopted from the glycolysis pathway in the labeled carbon isotope travel to different carbon positions through the network of reactions. The network starts with fructose 6-phosphateFructose 6-phosphate
Fructose 6-phosphate is fructose sugar phosphorylated on carbon 6 . The β-D-form of this compound is very common in cells. The vast majority of glucose and fructose entering a cell will become converted to this at some point...
(F6P) which has 6 carbon atoms with a label 13C at carbon position 1 and 2. F6P becomes T3P in which the labeled 1,2-13C F6P produces a 2,3-13C T3P. The 2,3-13C T3P can now introduce a different labeled F6P, a 5,6-13C F6P. The figure shows the use of stable isotope labeling to discover the carbon rearrangement through reactions using position specific labeled compounds.
Metabolic flux analysis using stable isotope labeling
Metabolic flux analysisMetabolic flux analysis
Metabolic flux analysis is an analysis technique similar to Flux Balance Analysis used to determine the rate at which a metabolite is produced during a bioprocess....
(MFA) using stable isotope labeling is an important tool to figuring out the metabolic pathways and reactions that occur within a cell. An isotopic label is fed to the cell, then the cell is allowed to grow, and finally the isotope pattern of the output metabolite is determined. The output isotope pattern provides valuable information which can be used to find the amount of flux
Flux
In the various subfields of physics, there exist two common usages of the term flux, both with rigorous mathematical frameworks.* In the study of transport phenomena , flux is defined as flow per unit area, where flow is the movement of some quantity per time...
, rate of conversion from reactants to products, through each reaction.
Figure 1 demonstrates the ability to use different labels to determine the flux through a certain reaction. Let us assume, the original metabolite
Metabolite
Metabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
, a three carbon metabolite, has the ability to split into a two carbon and one carbon metabolite, then recombine or remain a three carbon metabolite. If the reaction is provided with two isotopes of the metabolite in equal proportion, one completely labeled, commonly known as uniformly labeled, and one unlabeled. The pathway down the left side of the diagram does not display any change in the metabolites, while the right side shows the split and recombination. As shown, if the metabolite only takes the pathway down the left side, it remains in a 50-50 ratio of uniformly labeled to unlabeled metabolite. If the metabolite only takes the right side new labeling patterns can occur, all in equal proportion. Other proportions can occur depending on how much of the original metabolite follows the left side of the pathway versus the right side of the pathway. Here the proportions are shown for a situation in which half of the metabolites take the left side and half the right, but other proportions can occur. By measuring the proportion of the differently labeled metabolites, the flux through each reaction can be determined.
MFA results in a flux map which shows the amount of reactants being converted to products for each reaction. In most flux maps, the thicker the arrow, the larger the flux value of the reaction.
Figure 2 is an example of the MFA of the conversion of Glucose to Pyruvate. This is an example of a more complex flux map in comparison to Figure 1. In this map the tracking of the isotope is useful in figuring out what genes are needed to be added to maximize the production of a specific product.
Testing the isotopes
Any technique in measuring the difference between isotopomers can be used. The two primary methods, NMR and MS, have been developed for such purposes.Proton NMR was the first technique that was used for 13C-labeling experiments. Using this method, each single protonated carbon position inside a particular metabolite pool can be observed separately from the other positions.5 This allows the percentage of isotopomers labeled at that specific position to be known. The limit to proton NMR was that if there are n carbon atoms in a metabolite, there can only be at most n different positional enrichment values, which is only a small fraction of the total isotopomer information. Although the use of proton NMR labeling is limiting, pure proton NMR experiments are much easier to evaluate than experiments with more isotopomer information.
13C NMR spectrum allows a more detailed isotopomer distribution because a labeled carbon produces different hyperfine splitting signals depending on the labeling state of its direct neighbors in the molecule. 6. A singlet peak emerges if the neighboring carbons are not labeled. A doublet peak emerges if only one neighboring carbon is labeling. The size of the doublet split depends on the functional group of the neighboring carbon. If two neighboring carbons are labeled, a doublet of doublets may degenerate into a triplet if the doublet splittings are equal.
The drawbacks to using NMR techniques for Metabolic Flux Analysis purposes is that it is a rather specialized discipline which is different from other NMR applictions. An NMR spectrometer may not be directly available for all research teams. The optimization iof NMR measurement parameters and proper analysis of peak structures requires a skilled NMR specialist. Certain metabolites may require specialized measurement procedures to obtain additional isotopomer data.
In addition, specially adapted software tools are needed to determine the precie quantity of peak areas as well as identifying the decomposition of entangled singlet, doublet, and triplet peaks.
Another method as opposed to NMR is mass spectrometry which is more applicable and sensitive to MFA experiments. All MS instruments work directly with hydrolysate.
In GC-MS, the MS is couple to a gas chromatograph to separate the compounds of the hydrolysate.
The compounds eluting from the GC column are then ionized and simultaneously fragmented. The benefit in using GC-MS is that not only the mass isotopomers of the molecular ion are measured but also the mass isotopomer spectrum of several fragments, which significantly increases the measured information.
MS instruments divide a particular isotopomer distribution by its molecular weight, meaning that all isotopomers of a particular metabolite contains the same number of labeled carbon atoms are collected in one peak signal. Because every isotopomer contributes to exactly one peak in the MS spectrum, A percentage value can then be calculated for each peak, yielding the mass isotopomer fraction. 5
The drawbacks to using MS techniques is that the sample must be prepared by chemical derivation in order to obtain molecules with charge. In addition, the retention time of differently labeled isotopomers in the GC column also depends on the isotopomer. The natural abundance of other atoms than carbon also leads to a disturbance in the mass isotopomer spectrum.
Radioisotopic labeling
Radioisotopic labeling is a technique for tracking the passage of a sample of substance through a system. The substance is "labeled" by including radionuclideRadionuclide
A radionuclide is an atom with an unstable nucleus, which is a nucleus characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or to an atomic electron. The radionuclide, in this process, undergoes radioactive decay, and emits gamma...
s in its chemical composition. When these decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
, their presence can be determined by detecting the radiation
Particle radiation
Particle radiation is the radiation of energy by means of fast-moving subatomic particles. Particle radiation is referred to as a particle beam if the particles are all moving in the same direction, similar to a light beam....
emitted by them. Radioisotopic labeling is a special case of isotopic labeling.
For these purposes, a particularly useful type of radioactive decay is positron emission
Positron emission
Positron emission or beta plus decay is a type of beta decay in which a proton is converted, via the weak force, to a neutron, releasing a positron and a neutrino....
. When a positron collides with an electron, it releases two high-energy photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s traveling in diametrically opposite directions. If the positron is produced within a solid object, it is likely to do this before traveling more than a millimeter. If both of these photons can be detected, the location of the decay event can be determined very precisely.
Strictly speaking, radioisotopic labeling includes only cases where radioactivity is artificially introduced by experimenters, but some natural phenomena allow similar analysis to be performed. In particular, radiometric dating
Radiometric dating
Radiometric dating is a technique used to date materials such as rocks, usually based on a comparison between the observed abundance of a naturally occurring radioactive isotope and its decay products, using known decay rates...
uses a closely related principle.
Isotopic tracer

Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
and biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
to help understand chemical reactions
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
and interactions. In this technique, one or more of the atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s of the molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
of interest is substituted for an atom of the same chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
, but of a different (often radioactive, such as in radioactive tracing
Radioactive tracer
A radioactive tracer, also called a radioactive label, is a substance containing a radioisotope that is used to measure the speed of chemical processes and to track the movement of a substance through a natural system such as a cell or tissue...
) isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
. Because the atom has the same number of protons, it will behave in almost exactly the same way chemically as other atoms in the compound, and with few exceptions will not interfere with the reaction under investigation. The difference in the number of neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s, however, means that it can be detected separately from the other atoms of the same element.
NMR
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
typically uses this type of technique to investigate the mechanisms of chemical reactions (basically trying to find out which starting atom ends up where after a reaction), because NMR detects not only isotopic differences, but also gives an indication of the position of the atom.
Mass spectrometry can also be used with this technique, since mass spectra recorded with sufficiently high resolution can distinguish among isotopes based on the different masses resulting from the different number of neutrons.
Autoradiograph
Autoradiograph
An autoradiograph is an image on an x-ray film or nuclear emulsion produced by the pattern of decay emissions from a distribution of a radioactive substance...
s of gels in gel electrophoresis
Gel electrophoresis
Gel electrophoresis is a method used in clinical chemistry to separate proteins by charge and or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge...
can also take advantage of this approach. In this technique, radioactive isotopes are used. The radiation emitted by compounds containing the radioactive isotopes darkens a piece of photographic film
Photographic film
Photographic film is a sheet of plastic coated with an emulsion containing light-sensitive silver halide salts with variable crystal sizes that determine the sensitivity, contrast and resolution of the film...
, recording the position of these compounds relative to one another in the gel.
Isotopic (isotope) tracers are some of the most important tools in geology
Geology
Geology is the science comprising the study of solid Earth, the rocks of which it is composed, and the processes by which it evolves. Geology gives insight into the history of the Earth, as it provides the primary evidence for plate tectonics, the evolutionary history of life, and past climates...
, because they can be used to understand complex mixing process in earth systems. Isotope tracers are almost always used in the form of isotope ratios. By studying the ratio between two isotopes of the same element, we avoid effects involving the overall abundance of the element, which would usually swamp the much smaller variations in isotopic abundances. Further discussion of the application of isotopic tracers in geology is covered under the heading of isotope geochemistry
Isotope geochemistry
Isotope geochemistry is an aspect of geology based upon study of the relative and absolute concentrations of the elements and their isotopes in the Earth. Variations in the abundance of these isotopes, typically measured with an isotope ratio mass spectrometer or an accelerator mass spectrometer,...
.
Isotopic tracers are usually subdivided into two categories: radiogenic isotope tracers and stable isotope
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
tracers. A radiogenic isotope tracer involves an isotope produced by radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
, which is usually ratioed against a non-radiogenic isotope (whose abundance in the earth does not vary due to radioactive decay).
A stable isotope tracer involves only non-radiogenic isotopes. In this case, relative variations in abundance between the two isotopes is most often caused by mass-dependent fractionation. In theory, any element with two stable isotopes can be used as an isotopic tracer. However, the most commonly used stable isotope tracers involve relatively light isotopes which readily undergo fractionation in natural systems. See also isotopic signature
Isotopic signature
An isotopic signature is a ratio of stable or unstable isotopes of particular elements found in an investigated material...
.
Other uses in metabolic engineering
In Proteomics the branch of genetic that studies the full set of protein expressed by a genome, identifying diseases biomarker, for example, involves the usage of Stable isotope labeling by amino acids in cell culture (SILAC), that provides isotopic label forms of amino acid which help estimating protein levels. In protein recombinant, manipulated proteins are produced in large quantities to be used in useful products. Isotope labeling techniques in protein recombinant that help testing for relevant proteins, used to be about selectively enrich nuclei with 13C or 15N or deplete 1H from them. The recombinant would be expressed in E.coli with media containing 15N-ammonium chloride as a source of nitrogen. The resulting 15N labeled proteins are then purified by immobilized metal affinity and its percentage estimated. In the desire to increase the yield for a low isotope cost, a different procedure primarily increases the cell mass using unlabeled media before introducing it in a minimal amount of labeled media. Other labeling methods for measuring DNA synthesis that is cell proliferation in vitro uses H3-thymidine labeling to compare pattern of synthesis (or sequence) in cells.Applications for Oceanography
Isotopic tracers are used extensively in oceanography to study a wide array of processes. The isotopes used are typically naturally occurring with well-established sources and rates of formation and decay. However, anthropogenic isotopes may also be used with great success. The researchers measure the isotopic ratios at different locations and times to infer information about the physical processes of the ocean.Particle Transport
The ocean is an extensive network of particle transport. Thorium isotopes can help researchers decipher the vertical and horizontal movement of matter. 223Th has a constant, well-defined production rate in the ocean and a half-life of 24 days. This naturally occurring isotope has been shown to vary linearly with depth. Therefore, any changes in this linear pattern can be attributed to the transport of 223Th on particles. For example, low isotopic ratios in surface water with very high values a few meters down would indicate a vertical flux in the downward direction. Furthermore, the thorium isotope may be traced within a specific depth to decipher the lateral transport of particles.Circulation
Circulation within local systems, such as bays, estuaries, and groundwater, may be examined with radium isotopes. 223Ra has a half-life of 11 days and can occur naturally at specific locations in rivers and groundwater sources. The isotopic ratio of radium will then decrease as the water from the source river enters a bay or estuary. By measuring the amount of 223Ra at a number of different locations, a circulation pattern can be deciphered. This same exact process can also be used to study the movement and discharge of groundwater.Various isotopes of lead can be used to study circulation on a global scale. Different oceans (i.e. the Atlantic, Pacific, Indian, etc.) have different isotopic signatures. This results from differences in isotopic ratios of sediments and rocks within the different oceans. Because the different isotopes of lead have half-lives of 50-200 years, there is not enough time for the isotopic ratios to be homogenized throughout the whole ocean. Therefore, precise analysis of Pb isotopic ratios can be used to study the circulation of the different oceans.
Tectonic Processes and Climate Change
Isotopes with extremely long half-lives can be used to study multi-million year processes, such as tectonics and extreme climate change. The isotopic ratio of strontium (half-life ~2 Ma) can be analyzed within ice cores to examine changes over the earth’s lifetime. Differences in this ratio within the ice core would indicate significant alterations in the earth’s geochemistry.Isotopes Related to Nuclear Weapons
The aforementioned processes can be measured using naturally occurring isotopes. Nevertheless, anthropogenic isotopes are also extremely useful for oceanographic measurements. Nuclear weapons tests released a plethora of uncommon isotopes into the world’s oceans. 3H, 129I, and 137Cs can be found dissolved in seawater, while 241Am and 238Pu are attached to particles. The isotopes dissolved in water are particularly useful in studying global circulation. For example, differences in lateral isotopic ratios within an ocean can indicate strong water fronts or gyres. Conversely, the isotopes attached to particles can be used to study mass transport within water columns. For instance, high levels of Am or Pu can indicate downwelling when observed at great depths, or upwelling when observed at the surface.Methods for isotopic labeling
- Chemical synthesis
- Enzyme-mediated exchange
- Recombinant protein expression in isotopic labeled media.
See also
- Uses of radionuclides
- Radioactivity in biology
- Radioactive tracerRadioactive tracerA radioactive tracer, also called a radioactive label, is a substance containing a radioisotope that is used to measure the speed of chemical processes and to track the movement of a substance through a natural system such as a cell or tissue...
- Isotopomer
- isotopologueIsotopologueIsotopologues are molecules that differ only in their isotopic composition. Simply, the isotopologue of a chemical species has at least one atom with a different number of neutrons than the parent....

