
Isomers of butylene
Encyclopedia
Butene, also known as butylene, is an alkene
with the formula C
4H
8. It is a colourless gas that is present in crude oil as a minor constituent in quantities that are too small for viable extraction. It is therefore obtained by catalytic cracking
of long chain hydrocarbons left during refining of crude oil. Cracking produces a mixture of products and the butene is extracted from this by fractional distillation
.
Butene can be used as the monomer
for polybutene but this polymer
is more expensive than alternatives with shorter carbon chains such as polypropylene
. Polybutene is therefore commonly used as a co-polymer (mixed with another polymer, either during or after reaction), such as in hot-melt adhesives.
48 four isomers are alkenes. All four of these hydrocarbons have four carbon
atom
s and one double bond
in their molecule
s, but have different chemical structure
s. The IUPAC and common names, respectively, of these chemical compound
s are:
In the chemical structures above, the small blue numbers in the structure images are the numbering of the atoms in the main backbone chain of the molecules. Other organic compounds have the formula C4H8, namely cyclobutane
and methylcyclopropane
, but are not alkenes and are not discussed here. There are also cyclic alkenes with four carbon atoms overall such as cyclobutene
and two isomers of methylcyclopropene, but they do not have the formula C4H8 and are not discussed here.
All four of these isomers are gas
es at room temperature
and pressure
, but can be liquefied by lowering the temperature or raising the pressure on them, in a manner similar to pressurised butane
. These gases are colourless, but do have distinct odours, and are highly flammable. Although not naturally present in petroleum
in high percentages, they can be produced from petrochemicals or by catalytic cracking
of petroleum
. Although they are stable compounds, the carbon-carbon double bonds make them more reactive than similar alkanes, which are more inert compounds in various ways.
Because of the double bonds, these 4-carbon alkenes can act as monomers in the formation of polymers, as well as having other uses as petrochemical
intermediates. They are used in the production of synthetic rubber
. But-1-ene is a linear or normal alpha-olefin
and isobutylene is a branched alpha-olefin. In a rather low percentage, but-1-ene is used as one of the comonomers, along with other alpha-olefins, in the production of high density polyethylene
and linear low density polyethylene
. Butyl rubber
is made by cationic polymerisation of isobutylene with about 2 - 7% isoprene
. Isobutylene is also used for the production of methyl tert-butyl ether (MTBE) and isooctane, both of which improve the combustion of gasoline
.
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
with the formula C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
4H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
8. It is a colourless gas that is present in crude oil as a minor constituent in quantities that are too small for viable extraction. It is therefore obtained by catalytic cracking
Fluid catalytic cracking
Fluid catalytic cracking is the most important conversion process used in petroleum refineries. It is widely used to convert the high-boiling, high-molecular weight hydrocarbon fractions of petroleum crude oils to more valuable gasoline, olefinic gases, and other products...
of long chain hydrocarbons left during refining of crude oil. Cracking produces a mixture of products and the butene is extracted from this by fractional distillation
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
.
Butene can be used as the monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
for polybutene but this polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
is more expensive than alternatives with shorter carbon chains such as polypropylene
Polypropylene
Polypropylene , also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging, textiles , stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes...
. Polybutene is therefore commonly used as a co-polymer (mixed with another polymer, either during or after reaction), such as in hot-melt adhesives.
Isomers
Among the molecules which have the chemical formulaChemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
48 four isomers are alkenes. All four of these hydrocarbons have four carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s and one double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
in their molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s, but have different chemical structure
Chemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
s. The IUPAC and common names, respectively, of these chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s are:
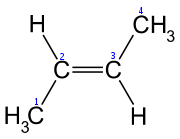
1-Butene 1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air... |
 |
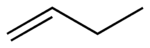 |
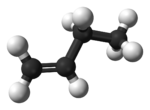 |
|
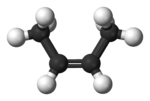
2-Butene 2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil... |
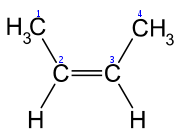 |
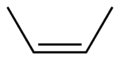 |
 |
|
2-Butene 2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil... |
 |
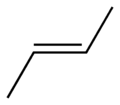 |
 |
|
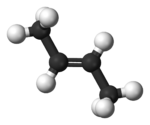
Isobutylene Isobutylene is a hydrocarbon of significant industrial importance. It is a four-carbon branched alkene , one of the four isomers of butylene. At standard temperature and pressure it is a colorless flammable gas.-Uses:... |
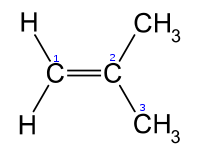 |
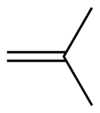 |
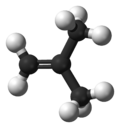 |
|
In the chemical structures above, the small blue numbers in the structure images are the numbering of the atoms in the main backbone chain of the molecules. Other organic compounds have the formula C4H8, namely cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
and methylcyclopropane
Methylcyclopropane
Methylcyclopropane is an organic compound with the structural formula C3H5CH3. This colorless gas is the monomethyl derivative of cyclopropane.- Reactions :...
, but are not alkenes and are not discussed here. There are also cyclic alkenes with four carbon atoms overall such as cyclobutene
Cyclobutene
Cyclobutene is a cycloalkene. It is used in the chemical industry as a monomer for synthesis of some polymers and for a range of chemical syntheses.- External links :*...
and two isomers of methylcyclopropene, but they do not have the formula C4H8 and are not discussed here.
All four of these isomers are gas
Gas
Gas is one of the three classical states of matter . Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point , boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons...
es at room temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, but can be liquefied by lowering the temperature or raising the pressure on them, in a manner similar to pressurised butane
Butane
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
. These gases are colourless, but do have distinct odours, and are highly flammable. Although not naturally present in petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
in high percentages, they can be produced from petrochemicals or by catalytic cracking
Cracking (chemistry)
In petroleum geology and chemistry, cracking is the process whereby complex organic molecules such as kerogens or heavy hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products...
of petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
. Although they are stable compounds, the carbon-carbon double bonds make them more reactive than similar alkanes, which are more inert compounds in various ways.
Because of the double bonds, these 4-carbon alkenes can act as monomers in the formation of polymers, as well as having other uses as petrochemical
Petrochemical
Petrochemicals are chemical products derived from petroleum. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as corn or sugar cane....
intermediates. They are used in the production of synthetic rubber
Synthetic rubber
Synthetic rubber is is any type of artificial elastomer, invariably a polymer. An elastomer is a material with the mechanical property that it can undergo much more elastic deformation under stress than most materials and still return to its previous size without permanent deformation...
. But-1-ene is a linear or normal alpha-olefin
Alpha-olefin
Alpha-olefins are a family of organic compounds which are olefins or alkenes with a chemical formula CxH2x, distinguished by having a double bond at the primary or alpha position...
and isobutylene is a branched alpha-olefin. In a rather low percentage, but-1-ene is used as one of the comonomers, along with other alpha-olefins, in the production of high density polyethylene
High density polyethylene
High-density polyethylene or polyethylene high-density is a polyethylene thermoplastic made from petroleum. It takes 1.75 kilograms of petroleum to make one kilogram of HDPE...
and linear low density polyethylene
Linear low density polyethylene
Linear low-density polyethylene is a substantially linear polymer , with significant numbers of short branches, commonly made by copolymerization of ethylene with longer-chain olefins. Linear low-density polyethylene differs structurally from conventional low-density polyethylene because of the...
. Butyl rubber
Butyl rubber
Butyl rubber is a synthetic rubber, a copolymer of isobutylene with isoprene. The abbreviation IIR stands for Isobutylene Isoprene Rubber. Polyisobutylene, also known as "PIB" or polyisobutene, n, is the homopolymer of isobutylene, or 2-methyl-1-propene, on which butyl rubber is based...
is made by cationic polymerisation of isobutylene with about 2 - 7% isoprene
Isoprene
Isoprene , or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=CCH=CH2. Under standard conditions it is a colorless liquid...
. Isobutylene is also used for the production of methyl tert-butyl ether (MTBE) and isooctane, both of which improve the combustion of gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
.

