
Inversion temperature
Encyclopedia
The inversion temperature in thermodynamics
and cryogenics
is the critical temperature below which a non-ideal gas
(all gases in reality) that is expanded at constant enthalpy will experience a temperature decrease, and above which will experience a temperature increase. This temperature change is known as the Joule-Thomson effect
, and is exploited in the liquefaction of gases
.
cannot be described in the theory of ideal gases, in which interactions between particles are ignored. Instead, one must use a theory that accounts for the Van der Waals force
between interacting particles that becomes much stronger as a gas becomes a liquid.
For a van der Waals gas
we can calculate the enthalpy
H using statistical mechanics
as
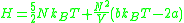 ,
,
where is the number of molecules,
is the number of molecules,  is volume,
is volume, 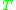 is temperature (in the Kelvin scale),
is temperature (in the Kelvin scale),  is Boltzmann's constant, and
is Boltzmann's constant, and  and
and 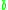 are constants depending on intermolecular forces and molecular volume, respectively.
are constants depending on intermolecular forces and molecular volume, respectively.
From this equation, we note that if we keep enthalpy constant and increase volume, temperature must change depending on the sign of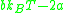 . Therefore, our inversion temperature is given where the sign flips at zero, or
. Therefore, our inversion temperature is given where the sign flips at zero, or
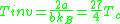 ,
,
where is the critical temperature of the substance. So for
is the critical temperature of the substance. So for  , an expansion at constant enthalpy increases temperature as the work
, an expansion at constant enthalpy increases temperature as the work
done by the repulsive interactions of the gas is dominant, and so the change in energy
is negative. But for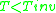 , expansion causes temperature to decrease because the work of attractive intermolecular forces dominates, giving a positive change in energy.
, expansion causes temperature to decrease because the work of attractive intermolecular forces dominates, giving a positive change in energy.
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
and cryogenics
Cryogenics
In physics, cryogenics is the study of the production of very low temperature and the behavior of materials at those temperatures. A person who studies elements under extremely cold temperature is called a cryogenicist. Rather than the relative temperature scales of Celsius and Fahrenheit,...
is the critical temperature below which a non-ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
(all gases in reality) that is expanded at constant enthalpy will experience a temperature decrease, and above which will experience a temperature increase. This temperature change is known as the Joule-Thomson effect
Joule-Thomson effect
In thermodynamics, the Joule–Thomson effect or Joule–Kelvin effect or Kelvin–Joule effect describes the temperature change of a gas or liquid when it is forced through a valve or porous plug while kept insulated so that no heat is exchanged with the environment. This procedure is called a...
, and is exploited in the liquefaction of gases
Liquefaction of gases
Liquefaction of gases includes a number of phases used to convert a gas into a liquid state. The processes are used for scientific, industrial and commercial purposes. Many gases can be put into a liquid state at normal atmospheric pressure by simple cooling; a few, such as carbon dioxide, require...
.
Theory
The Joule-Thomson effectJoule-Thomson effect
In thermodynamics, the Joule–Thomson effect or Joule–Kelvin effect or Kelvin–Joule effect describes the temperature change of a gas or liquid when it is forced through a valve or porous plug while kept insulated so that no heat is exchanged with the environment. This procedure is called a...
cannot be described in the theory of ideal gases, in which interactions between particles are ignored. Instead, one must use a theory that accounts for the Van der Waals force
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
between interacting particles that becomes much stronger as a gas becomes a liquid.
For a van der Waals gas
Van der Waals equation
The van der Waals equation is an equation of state for a fluid composed of particles that have a non-zero volume and a pairwise attractive inter-particle force It was derived by Johannes Diderik van der Waals in 1873, who received the Nobel prize in 1910 for "his work on the equation of state for...
we can calculate the enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
H using statistical mechanics
Statistical mechanics
Statistical mechanics or statistical thermodynamicsThe terms statistical mechanics and statistical thermodynamics are used interchangeably...
as
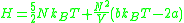 ,
,where
 is the number of molecules,
is the number of molecules,  is volume,
is volume, 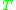 is temperature (in the Kelvin scale),
is temperature (in the Kelvin scale),  is Boltzmann's constant, and
is Boltzmann's constant, and  and
and 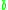 are constants depending on intermolecular forces and molecular volume, respectively.
are constants depending on intermolecular forces and molecular volume, respectively.From this equation, we note that if we keep enthalpy constant and increase volume, temperature must change depending on the sign of
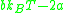 . Therefore, our inversion temperature is given where the sign flips at zero, or
. Therefore, our inversion temperature is given where the sign flips at zero, or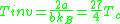 ,
,where
 is the critical temperature of the substance. So for
is the critical temperature of the substance. So for  , an expansion at constant enthalpy increases temperature as the work
, an expansion at constant enthalpy increases temperature as the workWork (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
done by the repulsive interactions of the gas is dominant, and so the change in energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
is negative. But for
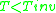 , expansion causes temperature to decrease because the work of attractive intermolecular forces dominates, giving a positive change in energy.
, expansion causes temperature to decrease because the work of attractive intermolecular forces dominates, giving a positive change in energy.External links
- Thermodynamic Concepts and Processes (Chapter 2) (part of the Statistical and Thermal Physics (STP) Curriculum Development Project at Clark UniversityClark UniversityClark University is a private research university and liberal arts college in Worcester, Massachusetts.Founded in 1887, it is the oldest educational institution founded as an all-graduate university. Clark now also educates undergraduates...
)

