
Inner sphere electron transfer
Encyclopedia
Inner sphere or bonded electron transfer proceeds via a covalent linkage between the two redox partners, the oxidant and the reductant. In Inner Sphere (IS) electron transfer (ET), a ligand
bridges the two metal redox
centers during the electron transfer event. Inner sphere reactions are inhibited by large ligands, which prevent the formation of the crucial bridged intermediate. Thus, IS ET is rare in biological systems, where redox sites are often shielded by bulky proteins. Inner sphere ET is usually used to describe reactions involving transition metal complexes and most of this article is written from this perspective. However, redox centers can consist of organic groups rather than metal centers.
The bridging ligand
could be virtually any entity that can convey electrons. Typically, such a ligand has more than one lone electron pair, such that it can serve as an electron donor to both the reductant and the oxidant. Common bridging ligands include the halides and the pseudohalides such as hydroxide
and thiocyanate
. More complex bridging ligands are also well known including oxalate
, malonate, and pyrazine
. Prior to ET, the bridged complex must form, and such processes are often highly reversible. Electron transfer occurs through the bridge once it is established. In some cases, the stable bridged structure may exist in the ground state; in other cases, the bridged structure may be a transiently-formed intermediate, or else as a transition state during the reaction.
The alternative to inner sphere electron transfer is outer sphere electron transfer
. In any transition metal redox process, the mechanism can be assumed to be outer sphere unless the conditions of the inner sphere are met. Inner sphere electron transfer is generally enthalpically
more favorable than outer sphere electron transfer due to a larger degree of interaction between the metal centers involved, however, inner sphere electron transfer is usually entropically
less favorable since the two sites involved must become more ordered (come together via a bridge) than in outer sphere electron transfer.
, who was awarded the Nobel Prize in Chemistry
in 1983 for his pioneering studies. A particularly historic finding is summarized in the abstract of the seminal publication.
“When Co(NH3)5Cl++ is reduced by Cr++ in M {meaning 1M} HClO4, 1 appears attached to Cr for each Cr(III) which is formed or Co(III) reduced. When the reaction is carried on in a medium containing radioactive Cl, the mixing of the attached to Cr(III) with that in solution is less than 0.5%. This experiment shows that transfer of Cl to the reducing agent from the oxidizing agent is direct…” The paper and the excerpt above can be described with the following equation:
The point of interest is that the chloride that was originally bonded to the cobalt, the oxidant, becomes bonded to chromium, which in its +3 oxidation state, forms kinetically inert bonds to its ligand
s. This observation implies the intermediacy of the bimetallic complex [Co(NH3)5(μ-Cl)Cr(H2O)5]4+, wherein "μ-Cl" indicates that the chloride bridges between the Cr and Co atoms, serving as a ligand for both. This chloride serves as a conduit for electron flow from Cr(II) to Co(III), forming Cr(III) and Co(II).
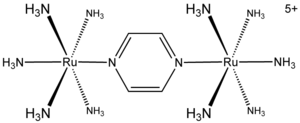 In the preceding example, the occurrence of the chloride bridge is inferred from the product analysis, but it was not observed. One complex that serves as a model for the bridged intermediate is the "Creutz Taube complex," [(NH3)5RuNC4H4NRu(NH3)5]5+. This species is named after Carol Creutz, who prepared the ion during her PhD studies with Henry Taube
In the preceding example, the occurrence of the chloride bridge is inferred from the product analysis, but it was not observed. One complex that serves as a model for the bridged intermediate is the "Creutz Taube complex," [(NH3)5RuNC4H4NRu(NH3)5]5+. This species is named after Carol Creutz, who prepared the ion during her PhD studies with Henry Taube
. The bridging ligand is the heterocycle pyrazine
, 1,4-C4H4N2. In the Creutz-Taube Ion, the average oxidation state of Ru is 2.5+. Spectroscopic
studies, however, show that the two Ru centers are equivalent, which indicates the ease with which the electron hole communicates between the two metals. The significance of the Creutz-Taube ion is its simplicity, which facilitates theoretical analysis, and its high symmetry, which ensures a high degree of delocalization. Many more complex mixed valence species are known both as molecules and polymeric materials.
which is present in more than one oxidation state
. Well-known mixed valence compounds include the Creutz-Taube complex
, Prussian blue
and Molybdenum blue
. Many solids are mixed-valency including indium chalcogenides
. Mixed valency is required for organic metals to exhibit electrical conductivity.
As the extinction coefficient decreases, the coupling constant decreases, influencing the angle to increase.
Mixed-valence compounds are subdivided into three groups, according to the Robin-Day Classification:
Organic mixed valence compounds are also known. Examples are the oxidized form of tetrathiafulvalene
and the radical cation of N,N,N',N'-tetramethyl-p-phenylenediamine.
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
bridges the two metal redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
centers during the electron transfer event. Inner sphere reactions are inhibited by large ligands, which prevent the formation of the crucial bridged intermediate. Thus, IS ET is rare in biological systems, where redox sites are often shielded by bulky proteins. Inner sphere ET is usually used to describe reactions involving transition metal complexes and most of this article is written from this perspective. However, redox centers can consist of organic groups rather than metal centers.
The bridging ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
could be virtually any entity that can convey electrons. Typically, such a ligand has more than one lone electron pair, such that it can serve as an electron donor to both the reductant and the oxidant. Common bridging ligands include the halides and the pseudohalides such as hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
and thiocyanate
Thiocyanate
Thiocyanate is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates...
. More complex bridging ligands are also well known including oxalate
Oxalate
Oxalate , is the dianion with formula C2O42− also written 22−. Either name is often used for derivatives, such as disodium oxalate, 2C2O42−, or an ester of oxalic acid Oxalate (IUPAC: ethanedioate), is the dianion with formula C2O42− also written (COO)22−. Either...
, malonate, and pyrazine
Pyrazine
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2.Pyrazine is a symmetrical molecule with point group D2h. Derivatives like phenazine are well known for their antitumor, antibiotic and diuretic activity. Pyrazine is less basic in nature than pyridine, pyridazine...
. Prior to ET, the bridged complex must form, and such processes are often highly reversible. Electron transfer occurs through the bridge once it is established. In some cases, the stable bridged structure may exist in the ground state; in other cases, the bridged structure may be a transiently-formed intermediate, or else as a transition state during the reaction.
The alternative to inner sphere electron transfer is outer sphere electron transfer
Outer sphere electron transfer
Outer sphere refers to an electron transfer event that occurs between chemical species that remain separate intact before, during, and after the ET event. In contrast, for inner sphere electron transfer the participating redox sites undergoing ET become connected by a chemical bridge...
. In any transition metal redox process, the mechanism can be assumed to be outer sphere unless the conditions of the inner sphere are met. Inner sphere electron transfer is generally enthalpically
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
more favorable than outer sphere electron transfer due to a larger degree of interaction between the metal centers involved, however, inner sphere electron transfer is usually entropically
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
less favorable since the two sites involved must become more ordered (come together via a bridge) than in outer sphere electron transfer.
Taube's experiment
The discoverer of the inner sphere mechanism was Henry TaubeHenry Taube
Henry Taube, Ph.D, M.Sc, B.Sc, FRSC was a Canadian-born American chemist noted for having been awarded the 1983 Nobel Prize in Chemistry for "his work in the mechanisms of electron-transfer reactions, especially in metal complexes." He was the first Canadian-born chemist to win the Nobel Prize...
, who was awarded the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
in 1983 for his pioneering studies. A particularly historic finding is summarized in the abstract of the seminal publication.
“When Co(NH3)5Cl++ is reduced by Cr++ in M {meaning 1M} HClO4, 1 appears attached to Cr for each Cr(III) which is formed or Co(III) reduced. When the reaction is carried on in a medium containing radioactive Cl, the mixing of the attached to Cr(III) with that in solution is less than 0.5%. This experiment shows that transfer of Cl to the reducing agent from the oxidizing agent is direct…” The paper and the excerpt above can be described with the following equation:
- [CoCl(NH3)5]2+ + [Cr(H2O)6]2+ → [Co(NH3)5(H2O)]2+ + [CrCl(H2O)5]2+
The point of interest is that the chloride that was originally bonded to the cobalt, the oxidant, becomes bonded to chromium, which in its +3 oxidation state, forms kinetically inert bonds to its ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s. This observation implies the intermediacy of the bimetallic complex [Co(NH3)5(μ-Cl)Cr(H2O)5]4+, wherein "μ-Cl" indicates that the chloride bridges between the Cr and Co atoms, serving as a ligand for both. This chloride serves as a conduit for electron flow from Cr(II) to Co(III), forming Cr(III) and Co(II).
The Creutz-Taube ion

Henry Taube
Henry Taube, Ph.D, M.Sc, B.Sc, FRSC was a Canadian-born American chemist noted for having been awarded the 1983 Nobel Prize in Chemistry for "his work in the mechanisms of electron-transfer reactions, especially in metal complexes." He was the first Canadian-born chemist to win the Nobel Prize...
. The bridging ligand is the heterocycle pyrazine
Pyrazine
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2.Pyrazine is a symmetrical molecule with point group D2h. Derivatives like phenazine are well known for their antitumor, antibiotic and diuretic activity. Pyrazine is less basic in nature than pyridine, pyridazine...
, 1,4-C4H4N2. In the Creutz-Taube Ion, the average oxidation state of Ru is 2.5+. Spectroscopic
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
studies, however, show that the two Ru centers are equivalent, which indicates the ease with which the electron hole communicates between the two metals. The significance of the Creutz-Taube ion is its simplicity, which facilitates theoretical analysis, and its high symmetry, which ensures a high degree of delocalization. Many more complex mixed valence species are known both as molecules and polymeric materials.
Mixed valence compounds
mixed valence compounds contain an elementChemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
which is present in more than one oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
. Well-known mixed valence compounds include the Creutz-Taube complex
Creutz-Taube Complex
The Creutz-Taube ion is the metal complex with the formula Ru5]25+. This cationic species has been heavily studied in an effort to understand the intimate details of inner sphere electron transfer, that is, how electrons move from one metal complex to another...
, Prussian blue
Prussian blue
Prussian blue is a dark blue pigment with the idealized formula Fe718. Another name for the color Prussian blue is Berlin blue or, in painting, Parisian blue. Turnbull's blue is the same substance but is made from different reagents....
and Molybdenum blue
Molybdenum blue
Molybdenum blue is a term applied to:*reduced heteropolymolybdate complexes, polyoxometalates containing Mo, Mo, and a hetero atom such as phosphorus or silicon...
. Many solids are mixed-valency including indium chalcogenides
Indium chalcogenides
The indium chalcogenides include all compounds of indium with the chalcogen elements, oxygen, sulfur, selenium and tellurium. . The best characterised compounds are the In and In chalcogenides e.g...
. Mixed valency is required for organic metals to exhibit electrical conductivity.
As the extinction coefficient decreases, the coupling constant decreases, influencing the angle to increase.
Mixed-valence compounds are subdivided into three groups, according to the Robin-Day Classification:
- Class I, where the valences are "trapped," or localized on a single site, such as Pb3O4Red leadLead tetroxide, also called minium, red lead or triplumbic tetroxide, is a bright red or orange crystalline or amorphous pigment. Chemically, red lead is lead tetroxide, Pb3O4, or 2PbO·PbO2....
and antimony tetroxideAntimony tetroxideAntimony tetroxide is an inorganic compound with the formula Sb2O4. This material, which exists as the mineral cervantite, is white but reversibly yellows upon heating...
. There are distinct sites with different specific valances in the complex that cannot easily interconvert. - Class II, which are intermediate in character. There is some localization of distinct valances, but there is a low activation energyActivation energyIn chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
for their interconversion. Some thermal activation is required to induce electron transfer from one site to another via the bridge. These species exhibit an intense Intervalence charge transfer (IT) band, a broad intense absorption in the IR- or visible part of the spectrum, and also exhibit magnetic exchange coupling at low temperatures. There is an unequal coefficient of mixing wave functions. Which can be determined by extinction coefficient of metal-metal charge transfer energy This type of complex is common when metals are in different ligand fields. For example, Prussian bluePrussian bluePrussian blue is a dark blue pigment with the idealized formula Fe718. Another name for the color Prussian blue is Berlin blue or, in painting, Parisian blue. Turnbull's blue is the same substance but is made from different reagents....
is an iron(II,III)–cyanideCyanideA cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
complex in which there is an iron(II) atom surrounded by six carbon atoms of six cyanideCyanideA cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
ligands bridged to an iron(III) atom by their nitrogen ends. In the Turnbull's blue preparation, an iron(II) solution is mixed with an iron(III) cyanide (c-linked) complex. An electron-transfer reaction occurs via the cyanide ligands to give iron(III) associated with an iron(II)-cyanide complex. - Class III, wherein mixed valence is not distinguishable by spectroscopic methods as the valence is completely delocalized. The Creutz-Taube Ion is an example of this class of complexes. These species also exhibit an IT band. There is a 50% ground state wave function and 50% excited state wavefunction. This class is typical when ligand environment is similar or identical for each of the two metal sites in the complex. The bridging ligand needs to be very good at electron transfer, conjugation, and be easily reduced.
Organic mixed valence compounds are also known. Examples are the oxidized form of tetrathiafulvalene
Tetrathiafulvalene
Tetrathiafulvalene is a organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, 2, by replacement of four CH groups with sulfur atoms...
and the radical cation of N,N,N',N'-tetramethyl-p-phenylenediamine.

