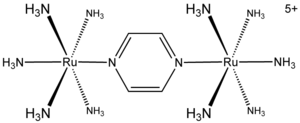
Creutz-Taube Complex
Encyclopedia
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
(NH3
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
)5]2(C4H4N2)5+. This cationic species has been heavily studied in an effort to understand the intimate details of inner sphere electron transfer
Inner sphere electron transfer
Inner sphere or bonded electron transfer proceeds via a covalent linkage between the two redox partners, the oxidant and the reductant. In Inner Sphere electron transfer , a ligand bridges the two metal redox centers during the electron transfer event. Inner sphere reactions are inhibited by...
, that is, how electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s move from one metal complex to another. The ion is named after Carol Creutz, who first prepared the complex, and her thesis advisor Henry Taube
Henry Taube
Henry Taube, Ph.D, M.Sc, B.Sc, FRSC was a Canadian-born American chemist noted for having been awarded the 1983 Nobel Prize in Chemistry for "his work in the mechanisms of electron-transfer reactions, especially in metal complexes." He was the first Canadian-born chemist to win the Nobel Prize...
, who received a Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
for this and related discoveries on electron-transfer.
Properties
The complex consists of two "pentammine" (ammonia in metal complexes is referred to as ammine) ruthenium unit linked to the nitrogen atoms in a bridging pyrazinePyrazine
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2.Pyrazine is a symmetrical molecule with point group D2h. Derivatives like phenazine are well known for their antitumor, antibiotic and diuretic activity. Pyrazine is less basic in nature than pyridine, pyridazine...
ligand, which completes the octahedral coordination sphere
Coordination sphere
In coordination chemistry, the coordination sphere refers to a central atom or ion and an array of molecules or anions, the ligands, around.Molecules that are attached noncovalently to the ligands are called the second coordination sphere....
of each metal. The important feature of the compound is that the two metals have apparent fractional oxidation states of 2.5+. Normally metal ions, like most ions, have integral oxidation states. For example, ruthenium ammines are typically 2+ or 3+. The fact that the oxidation states are half-integral indicates that the two Ru(NH3)5 centers are equivalent in terms of their number of electrons. Crystallographic and theoretical studies are consistent with this description, i.e. the two metals are equivalent. Characteristic of a mixed valence complex, this ion strongly absorbs light in the near-Infra-red part of the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
. In the case of the Creutz-Taube ion, the absorption maximum occurs at 1570 nm. This absorption is described as an intervalence charge-transfer band
Inter valence charge transfer
In chemistry, intervalence charge transfer, often abbreviated IVCT or even IT, is an electron transfer between two metal sites differing only in oxidation state. Quite often such electron transfer reverses the oxidation states of the sites...
.
Synthesis
The ion was originally isolated as the hydrated salt ([Ru(NH3)5]2(C4H4N2)(O3SC6H4CH3)5.3H2O). It is prepared in two steps from the monoruthenium pyrazine complex:- [Ru(NH3)5(C4H4N2)]2+ + [Ru(NH3)5(H2O)]2+ → [Ru(NH3)5]2(C4H4N2)]4+ + H2O
- [Ru(NH3)5]2(C4H4N2)]4+ + Ag+ → [Ru(NH3)5]2(C4H4N2)]5+ + Ag
The Creutz-Taube ion illustrates the advantages of ruthenium complexes for examining redox reactions. Ru(II) and Ru(III) ions can be interconverted at mild redox potentials
Standard electrode potential
In electrochemistry, the standard electrode potential, abbreviated E° or E , is the measure of individual potential of a reversible electrode at standard state, which is with solutes at an effective concentration of 1 mol dm−3, and gases at a pressure of 1 atm...
or using conventional redox reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
s. Importantly, coordination complexes of both of these oxidation states are kinetically inert, because they are both low-spin, d6 and d5, respectively. Many analogues of this ion have been prepared using different bridging ligands.

