Induced gamma emission
Encyclopedia
In physics
, induced gamma emission (IGE) refers to the process of fluorescent emission of gamma ray
s from excited nuclei, usually involving a specific nuclear isomer
. It is analogous to conventional fluorescence
, which is defined as the emission of a photon
(unit of light) by an excited electron in an atom or molecule. In the case of IGE, nuclear isomers can store significant amounts of excitation energy for times long enough for them to serve as nuclear fluorescent materials. There are over 800 known nuclear isomers but almost all are too intrinsically radioactive to be considered for applications. there were five proposed nuclear isomers that appeared to be physically capable of IGE fluorescence in safe arrangements: tantalum
-180m, osmium
-187m, platinum
-186m, hafnium
-178m2 and zinc
-66m.
 Induced gamma emission is an example of interdisciplinary research bordering on both nuclear physics and quantum electronics. Viewed as a nuclear reaction
Induced gamma emission is an example of interdisciplinary research bordering on both nuclear physics and quantum electronics. Viewed as a nuclear reaction
it would belong to a class in which only photons were involved in creating and destroying states of nuclear excitation. It is a class usually overlooked in traditional discussions. In 1939 Pontecorvo and Lazard reported the first example of this type of reaction. Indium
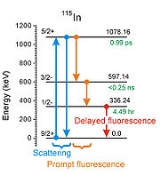
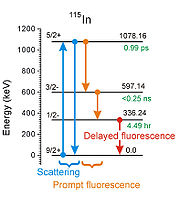
was the target and in modern terminology describing nuclear reactions it would be written 115In(γ,γ')115mIn. The product nuclide carries an "m" to denote that it has a long enough half life (4.5 hr in this case) to qualify as being a nuclear isomer
. That is what made the experiment possible in 1939 because the researchers had hours to remove the products from the irradiating environment and then to study them in a more appropriate location.
With projectile photons, momentum and energy can be conserved only if the incident photon, X-ray or gamma, has precisely the energy corresponding to the difference in energy between the initial state of the target nucleus and some excited state that is not too different in terms of quantum properties such as spin. There is no threshold behavior and the incident projectile disappears and its energy is transferred into internal excitation of the target nucleus. It is a resonant process that is uncommon in nuclear reactions but normal in the excitation of fluorescence at the atomic level. Only as recently as 1988 was the resonant nature of this type of reaction finally proven. Such resonant reactions are more readily described by the formalities of atomic fluorescence and further development was facilitated by an interdisciplinary approach of IGE.
There is little conceptual difference in an IGE experiment when the target is a nuclear isomer
. Such a reaction as mX(γ,γ')X where mX is one of the five candidates listed above, is only different because there are lower energy states for the product nuclide to enter after the reaction than there were at the start. Practical difficulties arise from the need to ensure safety from the spontaneous radioactive decay of nuclear isomers in quantities sufficient for experimentation. Lifetimes must be long enough that doses from the spontaneous decay from the targets always remain within safe limits. In 1988 Collins and coworkers reported the first excitation of IGE from a nuclear isomer. They excited fluorescence from the nuclear isomer
tantalum
-180m with x-rays produced by an external beam radiotherapy
"linac". Results were surprising and considered to be controversial until the resonant states excited in the target were identified. Fully independent confirmation was reported by the Stuttgart Nuclear Group in 1999.
field, where IGE is one of a number of theoretical "shortcuts" that are often discussed together. For instance, the apparently mythical red mercury
is another proposed mechanism to build a "mini-nuke", and it is not uncommon to see references to red mercury
as being either a ballotechnic
or IGE material. As a result, it is not uncommon to see confusion about ballotechnic materials being the same thing as IGE's.
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, induced gamma emission (IGE) refers to the process of fluorescent emission of gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s from excited nuclei, usually involving a specific nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
. It is analogous to conventional fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
, which is defined as the emission of a photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
(unit of light) by an excited electron in an atom or molecule. In the case of IGE, nuclear isomers can store significant amounts of excitation energy for times long enough for them to serve as nuclear fluorescent materials. There are over 800 known nuclear isomers but almost all are too intrinsically radioactive to be considered for applications. there were five proposed nuclear isomers that appeared to be physically capable of IGE fluorescence in safe arrangements: tantalum
Tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory...
-180m, osmium
Osmium
Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,...
-187m, platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
-186m, hafnium
Hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869. Hafnium was the penultimate stable...
-178m2 and zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
-66m.
History

Nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is semantically considered to be the process in which two nuclei, or else a nucleus of an atom and a subatomic particle from outside the atom, collide to produce products different from the initial particles...
it would belong to a class in which only photons were involved in creating and destroying states of nuclear excitation. It is a class usually overlooked in traditional discussions. In 1939 Pontecorvo and Lazard reported the first example of this type of reaction. Indium
Indium
Indium is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows the intermediate properties between these two...
was the target and in modern terminology describing nuclear reactions it would be written 115In(γ,γ')115mIn. The product nuclide carries an "m" to denote that it has a long enough half life (4.5 hr in this case) to qualify as being a nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
. That is what made the experiment possible in 1939 because the researchers had hours to remove the products from the irradiating environment and then to study them in a more appropriate location.
With projectile photons, momentum and energy can be conserved only if the incident photon, X-ray or gamma, has precisely the energy corresponding to the difference in energy between the initial state of the target nucleus and some excited state that is not too different in terms of quantum properties such as spin. There is no threshold behavior and the incident projectile disappears and its energy is transferred into internal excitation of the target nucleus. It is a resonant process that is uncommon in nuclear reactions but normal in the excitation of fluorescence at the atomic level. Only as recently as 1988 was the resonant nature of this type of reaction finally proven. Such resonant reactions are more readily described by the formalities of atomic fluorescence and further development was facilitated by an interdisciplinary approach of IGE.
There is little conceptual difference in an IGE experiment when the target is a nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
. Such a reaction as mX(γ,γ')X where mX is one of the five candidates listed above, is only different because there are lower energy states for the product nuclide to enter after the reaction than there were at the start. Practical difficulties arise from the need to ensure safety from the spontaneous radioactive decay of nuclear isomers in quantities sufficient for experimentation. Lifetimes must be long enough that doses from the spontaneous decay from the targets always remain within safe limits. In 1988 Collins and coworkers reported the first excitation of IGE from a nuclear isomer. They excited fluorescence from the nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
tantalum
Tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory...
-180m with x-rays produced by an external beam radiotherapy
External beam radiotherapy
External beam radiotherapy or teletherapy is the most common form of radiotherapy. The patient sits or lies on a couch and an external source of radiation is pointed at a particular part of the body...
"linac". Results were surprising and considered to be controversial until the resonant states excited in the target were identified. Fully independent confirmation was reported by the Stuttgart Nuclear Group in 1999.
Distinctive features
- If an incident photon is absorbed by an initial state of a target nucleus, that nucleus will be raised to a more energetic state of excitation. If that state can radiate its energy only during a transition back to the initial state, the result is a scattering process as seen in the schematic figure. That is not an example of IGE.
- If an incident photon is absorbed by an initial state of a target nucleus, that nucleus will be raised to a more energetic state of excitation. If there is a nonzero probability that sometimes that state will start a cascade of transitions as shown in the schematic, that state has been called a "gateway state" or "trigger level" or "intermediate state". One or more fluorescent photons are emitted, often with different delays after the initial absorption and the process is an example of IGE.
- If the initial state of the target nucleus is its ground (lowest energy) state, then the fluorescent photons will have less energy than that of the incident photon (as seen in the schematic figure). Since the scattering channel is usually the strongest, it can "blind" the instruments being used to detect the fluorescence and early experiments preferred to study IGE by pulsing the source of incident photons while detectors were gated off and then concentrating upon any delayed photons of fluorescence when the instruments could be safely turned back on.
- If the initial state of the target nucleus is a nuclear isomer (starting with more energy than the ground) it can also support IGE. However in that case the schematic diagram is not simply the example seen for 115In but read from right to left with the arrows turned the other way. Such a "reversal" would require simultaneous (to within <0.25 ns) absorption of two incident photons of different energies to get from the 4 hr isomer back up to the "gateway state". Usually the study of IGE from a ground state to an isomer of the same nucleus teaches little about how the same isomer would perform if used as the initial state for IGE. In order to support IGE an energy for an incident photon would have to be found that would "match" the energy needed to reach some other gateway state not shown in the schematic that could launch its own cascade down to the ground state.
- If the target is a nuclear isomer storing a considerable amount of energy then IGE might produce a cascade that contains a transition that emits a photon with more energy than that of the incident photon. This would be the nuclear analog of upconversionEnergy transfer upconversionEnergy Transfer Upconversion or ETU is a physical principle that involves the excitation of a laser-active ion to a level above that which would be achieved by simple absorption of a pump photon, the required additional energy being transferred from another laser-active ion undergoing nonradiative...
in laserLaserA laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
physics.
- If the target is a nuclear isomer storing a considerable amount of energy then IGE might produce a cascade through a pair of excited states whose lifetimes are "inverted" so that in a collection of such nuclei, population would build up in the longer lived upper level while emptying rapidly from the shorter lived lower member of the pair. The resulting inversion of population might support some form of coherent emission analogous to amplified spontaneous emissionAmplified spontaneous emissionAmplified spontaneous emission or superluminescence is light, produced by spontaneous emission, that has been optically amplified by the process of stimulated emission in a gain medium. It is inherent in the field of random lasers....
(ASE) in laser physicsLaser PhysicsLaser Physics is an international scientific journal published by Nauka/Interperiodica. It is distributed through the Springer.-Topics covered:The journal specializes in laser physics, but also publishes papers about:...
. If the physical dimensions of the collection of target isomer nuclei were long and thin, then a sort of "gamma ray laser" might result.
Potential applications
- Since the IGE from ground state nuclei requires the absorption of very specific photon energies to produce delayed fluorescent photons that are easily counted, there is the possibility to construct energy-specific dosimeters by combining several different nuclides. This was demonstrated for the calibration of the radiation spectrum from the DNA-PITHON pulsed nuclear simulator. Such a dosimeter could be useful in radiation therapyRadiation therapyRadiation therapy , radiation oncology, or radiotherapy , sometimes abbreviated to XRT or DXT, is the medical use of ionizing radiation, generally as part of cancer treatment to control malignant cells.Radiation therapy is commonly applied to the cancerous tumor because of its ability to control...
where X-ray beams may contain many energies. Since photons of different energies deposit their effects at different depths in the tissue being treated, it could help calibrate how much of the total dose would be deposited in the actual target volume.
- In February 2003, the non-peer reviewed New Scientist wrote about the possibility of an IGE-powered airplane. The idea was to utilize 178m2Hf (presumably due to its high energy to weight ratio) which would be triggered to release gamma rays that would heat air in a chamber for jet propulsion. This power source is apparently called a "quantum nucleonic reactor", although it is not clear if this name exists only in reference to the New Scientist article.

- It is partly this theoretical density that has made the entire IGE field so controversial. It has been suggested that the materials might be constructed to allow all of the stored energy to be released very quickly in a "burst". The density of gammas produced in this reaction would be high enough that it might allow them to be used to compress the fusionNuclear fusionNuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
fuel of a fusion bomb. If this turns out to be the case, it might allow a fusion bomb to be constructed with no fissile material inside (i.e. a pure fusion weaponPure fusion weaponA pure fusion weapon is a hypothetical hydrogen bomb design that does not need a fission "primary" explosive to ignite the fusion of deuterium and tritium, two heavy isotopes of hydrogen . Such a weapon would require no fissile material and would therefore be much easier to build in secret than...
), and it is the control of the fissile material and the means for making it that underlies most attempts to stop nuclear proliferationNuclear proliferationNuclear proliferation is a term now used to describe the spread of nuclear weapons, fissile material, and weapons-applicable nuclear technology and information, to nations which are not recognized as "Nuclear Weapon States" by the Treaty on the Nonproliferation of Nuclear Weapons, also known as the...
. In fact, the possible energy release of the gammas alone would make IGE a potential high power "explosive" on its own, or a potential radiological weaponRadiological weaponA radiological weapon or radiological dispersion device is any weapon that is designed to spread radioactive material with the intent to kill, and cause disruption upon a city or nation....
. Basic research remains in early stages but that has not deterred the worrying about these possibilities.
Societal concerns
Due to the possibility, no matter how remote, of IGE being used as a shortcut to, or analog of, a nuclear bomb, IGE has become a "hot topic" in the arms controlArms control
Arms control is an umbrella term for restrictions upon the development, production, stockpiling, proliferation, and usage of weapons, especially weapons of mass destruction...
field, where IGE is one of a number of theoretical "shortcuts" that are often discussed together. For instance, the apparently mythical red mercury
Red mercury
Red mercury is a 19th-century term for protiodide or iodide of mercury. It was commonly recommended for use as an antisyphilitic as late as 1913, most notably during the early years of the Tuskegee Syphilis Experiments. Taken orally, it caused hematemesis...
is another proposed mechanism to build a "mini-nuke", and it is not uncommon to see references to red mercury
Red mercury
Red mercury is a 19th-century term for protiodide or iodide of mercury. It was commonly recommended for use as an antisyphilitic as late as 1913, most notably during the early years of the Tuskegee Syphilis Experiments. Taken orally, it caused hematemesis...
as being either a ballotechnic
Ballotechnics
In chemistry, ballotechnics are a class of materials that undergo a chemical reaction when quickly subjected to extreme pressures. These pressures are of the order of tens of thousands of atmospheres, and the chemical reactions are initiated by shock waves transmitted through the material. The...
or IGE material. As a result, it is not uncommon to see confusion about ballotechnic materials being the same thing as IGE's.
External links
- "Scary Things Come in Small Packages", Washington Post article of 2004 by Sharon WeinbergerSharon WeinbergerSharon Weinberger is an American journalist and writer on defense and security issues. She is currently a Carnegie/Newhouse School Legal Reporting Fellow where her "project will examine a legally murky intersection between ethics and fraud in military contracting"...
- Hf-isomer Summary Page of Results, C.B. Collins, University of Texas, Dallas
- "Atomic Powered Global Hawk Jet Reving For Take-Off?", a SciScoop weblog entry
- Conflicting Results on a Long-Lived Nuclear Isomer of Hafnium Have Wider Implications This Physics Today article provides a balanced view from 2004.
- Reprints of articles about nuclear isomers in peer reviewed journals. - The Center for Quantum Electronics, The University of Texas at Dallas.

