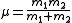Heavy Rydberg system
Encyclopedia
A heavy Rydberg system consists of a weakly bound positive and negative ion
orbiting their common centre of mass. Such systems share many properties with the conventional Rydberg atom
and consequently are sometimes referred to as heavy Rydberg atoms. While such a system is a type of ionically bound molecule, it should not be confused with a molecular Rydberg state, which is simply a molecule with one or more highly excited electrons.
The peculiar properties of the Rydberg atom come from the large charge separation and the resulting hydrogenic
potential. The extremely large separation between the two components of a heavy Rydberg system results in an almost perfect 1/r hydrogenic potential seen by each ion. The positive ion can be viewed as analogous to the nucleus of a hydrogen atom, with the negative ion playing the role of the electron.
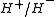 system, consisting of a proton bound with a
system, consisting of a proton bound with a 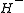 ion. The
ion. The 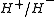 system was first observed in 2000 by a group at the University of Waterloo
system was first observed in 2000 by a group at the University of Waterloo
in Canada
.
The formation of the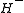 ion can be understood classically; as the single electron in a hydrogen atom
ion can be understood classically; as the single electron in a hydrogen atom
cannot fully shield the positively charged nucleus, another electron brought into close proximity will feel an attractive force. While this classical description is nice for getting a feel for the interactions involved, it is an oversimplification; many other atoms have a greater electron affinity
than hydrogen. In general the process of forming a negative ion is driven by the filling of atomic electron shells
to form a lower energy configuration.
Only a small number of molecules have been used to produce heavy Rydberg systems although in principle any atom with a positive electron affinity can bind with a positive ion. Species used include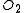 ,
, 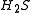 and
and 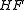 . Fluorine and oxygen are particularly favoured due to their high electron affinity, high ionisation energy
. Fluorine and oxygen are particularly favoured due to their high electron affinity, high ionisation energy
and consequently high electronegativity
.
in atoms) or rapid dissociation due to collisions or local fields
.
Currently production of heavy Rydberg systems relies on complex vacuum ultra-violet (so called because it is strongly absorbed in air and requires the entire system to be enclosed within a vacuum chamber) or multi-photon transitions (relying on absorption of multiple photons almost simultaneously), both of which are rather inefficient and result in systems with high internal energy.
in a heavy Rydberg system is 10,000 times larger than in a typical diatomic molecule
. As well as producing the characteristic hydrogen-like behaviour, this also makes them extremely sensitive to perturbation by external electric
and magnetic
fields.
Heavy Rydberg systems have a relatively large reduced mass
, given by:
This leads to a very slow time evolution, which makes them easy to manipulate both spatially and energetically, while their low binding energy
makes them relatively simple to detect through field dissociation and detection of the resulting ion
s, in a process known as threshold ion-pair production spectroscopy[1].
Kepler's third law
states that the period of an orbit is proportional to the cube of the semi-major axis
; this can be applied to the Coulomb force
:
where is the time-period,
is the time-period, 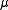 is the reduced mass,
is the reduced mass,  is the semi-major axis and
is the semi-major axis and 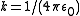 .
.
Classically we can say that a system with a large reduced mass has a long orbital period. Quantum mechanically, a large reduced mass in a system leads to narrow spacing of the energy level
s and the rate of time-evolution of the wavefunction
depends on this energy spacing. This slow time-evolution makes heavy Rydberg systems ideal for experimentally probing the dynamics of quantum systems.
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
orbiting their common centre of mass. Such systems share many properties with the conventional Rydberg atom
Rydberg atom
thumb|right|300px|Figure 1: Energy levels in atomic [[lithium]] showing the Rydberg series of the lowest 3 values of [[Angular momentum#Angular momentum in quantum mechanics|orbital angular momentum]] converging on the first ionization energy....
and consequently are sometimes referred to as heavy Rydberg atoms. While such a system is a type of ionically bound molecule, it should not be confused with a molecular Rydberg state, which is simply a molecule with one or more highly excited electrons.
The peculiar properties of the Rydberg atom come from the large charge separation and the resulting hydrogenic
Hydrogen-like atom
A hydrogen-like ion is any atomic nucleus with one electron and thus is isoelectronic with hydrogen. Except for the hydrogen atom itself , these ions carry the positive charge e, where Z is the atomic number of the atom. Examples of hydrogen-like ions are He+, Li2+, Be3+ and B4+...
potential. The extremely large separation between the two components of a heavy Rydberg system results in an almost perfect 1/r hydrogenic potential seen by each ion. The positive ion can be viewed as analogous to the nucleus of a hydrogen atom, with the negative ion playing the role of the electron.
Species of heavy Rydberg system
The most commonly studied system to date is the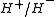 system, consisting of a proton bound with a
system, consisting of a proton bound with a 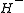 ion. The
ion. The 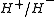 system was first observed in 2000 by a group at the University of Waterloo
system was first observed in 2000 by a group at the University of WaterlooUniversity of Waterloo
The University of Waterloo is a comprehensive public university in the city of Waterloo, Ontario, Canada. The school was founded in 1957 by Drs. Gerry Hagey and Ira G. Needles, and has since grown to an institution of more than 30,000 students, faculty, and staff...
in Canada
Canada
Canada is a North American country consisting of ten provinces and three territories. Located in the northern part of the continent, it extends from the Atlantic Ocean in the east to the Pacific Ocean in the west, and northward into the Arctic Ocean...
.
The formation of the
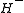 ion can be understood classically; as the single electron in a hydrogen atom
ion can be understood classically; as the single electron in a hydrogen atomHydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force...
cannot fully shield the positively charged nucleus, another electron brought into close proximity will feel an attractive force. While this classical description is nice for getting a feel for the interactions involved, it is an oversimplification; many other atoms have a greater electron affinity
Electron affinity
The Electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion....
than hydrogen. In general the process of forming a negative ion is driven by the filling of atomic electron shells
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
to form a lower energy configuration.
Only a small number of molecules have been used to produce heavy Rydberg systems although in principle any atom with a positive electron affinity can bind with a positive ion. Species used include
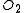 ,
, 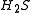 and
and 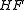 . Fluorine and oxygen are particularly favoured due to their high electron affinity, high ionisation energy
. Fluorine and oxygen are particularly favoured due to their high electron affinity, high ionisation energyIonization potential
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
and consequently high electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
.
Production of heavy Rydberg systems
The difficulty in the production of heavy Rydberg systems arises in finding an energetic pathway by which a molecule can be excited with just the right energy to form an ion pair, without sufficient internal energy to cause autodissociation (a process analogous to autoionizationAutoionization
Autoionization is a process by which atoms or molecules spontaneously emit one of the shell electrons, thus going from a state with charge Z to a state with charge Z + 1, for example from an electrically neutral state to a singly ionized state....
in atoms) or rapid dissociation due to collisions or local fields
Electromagnetic field
An electromagnetic field is a physical field produced by moving electrically charged objects. It affects the behavior of charged objects in the vicinity of the field. The electromagnetic field extends indefinitely throughout space and describes the electromagnetic interaction...
.
Currently production of heavy Rydberg systems relies on complex vacuum ultra-violet (so called because it is strongly absorbed in air and requires the entire system to be enclosed within a vacuum chamber) or multi-photon transitions (relying on absorption of multiple photons almost simultaneously), both of which are rather inefficient and result in systems with high internal energy.
What makes heavy Rydberg systems interesting?
The bond lengthBond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
in a heavy Rydberg system is 10,000 times larger than in a typical diatomic molecule
Diatomic
Diatomic molecules are molecules composed only of two atoms, of either the same or different chemical elements. The prefix di- means two in Greek. Common diatomic molecules are hydrogen , nitrogen , oxygen , and carbon monoxide . Seven elements exist in the diatomic state in the liquid and solid...
. As well as producing the characteristic hydrogen-like behaviour, this also makes them extremely sensitive to perturbation by external electric
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
and magnetic
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
fields.
Heavy Rydberg systems have a relatively large reduced mass
Reduced mass
Reduced mass is the "effective" inertial mass appearing in the two-body problem of Newtonian mechanics. This is a quantity with the unit of mass, which allows the two-body problem to be solved as if it were a one-body problem. Note however that the mass determining the gravitational force is not...
, given by:
This leads to a very slow time evolution, which makes them easy to manipulate both spatially and energetically, while their low binding energy
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
makes them relatively simple to detect through field dissociation and detection of the resulting ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s, in a process known as threshold ion-pair production spectroscopy[1].
Kepler's third law
Kepler's laws of planetary motion
In astronomy, Kepler's laws give a description of the motion of planets around the Sun.Kepler's laws are:#The orbit of every planet is an ellipse with the Sun at one of the two foci....
states that the period of an orbit is proportional to the cube of the semi-major axis
Semi-major axis
The major axis of an ellipse is its longest diameter, a line that runs through the centre and both foci, its ends being at the widest points of the shape...
; this can be applied to the Coulomb force
Coulomb's law
Coulomb's law or Coulomb's inverse-square law, is a law of physics describing the electrostatic interaction between electrically charged particles. It was first published in 1785 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism...
:
where
 is the time-period,
is the time-period, 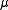 is the reduced mass,
is the reduced mass,  is the semi-major axis and
is the semi-major axis and 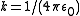 .
.Classically we can say that a system with a large reduced mass has a long orbital period. Quantum mechanically, a large reduced mass in a system leads to narrow spacing of the energy level
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
s and the rate of time-evolution of the wavefunction
Wavefunction
Not to be confused with the related concept of the Wave equationA wave function or wavefunction is a probability amplitude in quantum mechanics describing the quantum state of a particle and how it behaves. Typically, its values are complex numbers and, for a single particle, it is a function of...
depends on this energy spacing. This slow time-evolution makes heavy Rydberg systems ideal for experimentally probing the dynamics of quantum systems.