Glutamine synthetase
Encyclopedia
Glutamine synthetase (GS) is an enzyme
that plays an essential role in the metabolism
of nitrogen
by catalyzing the condensation of glutamate and ammonia
to form glutamine
:
Glutamate + ATP
+ NH3 → Glutamine + ADP
+ phosphate
Glutamine Synthetase uses ammonia produced by nitrate reduction, amino acid
degradation, and photorespiration
. The amide group of glutamate is a nitrogen source for the synthesis of glutamine pathway metabolites.
Other reactions may take place via GS. Competition between ammonium
ion and water, their binding affinities, and the concentration of ammonium ion, influences glutamine synthesis and glutamine hydrolysis. Glutamine is formed if an ammonium ion attacks the acyl-phosphate intermediate, while glutamate is remade if water attacks the intermediate. Ammonium ion binds more strongly than water to GS due to electrostatic forces between a cation and a negatively charged pocket. Another possible reaction is upon NH2OH binding to GS, rather than NH4+, yields γ-glutamylhydroxamate.
. Each active site creates a ‘bifunnel’ which is the site of three distinct substrate binding sites: nucleotide
, ammonium ion, and amino acid. ATP binds to the top of the bifunnel that opens to the external surface of GS. Glutamate binds at the bottom of the active site. The middle of the bifunnel contains two sites in which divalent cations bind (Mn+2 or Mg+2). One cation binding site is involved in phosphoryl transfer of ATP to glutamate, while the second stabilizes active GS and helps with the binding of glutamate.
Hydrogen bonding and hydrophobic interactions hold the two rings of GS together. Each subunit possesses a C-terminus and an N-terminus in its sequence. The C-terminus (helical thong) stabilizes the GS structure by inserting into the hydrophobic region of the subunit across in the other ring. The N-terminus is exposed to the solvent. In addition, the central channel is formed via six four-stranded β-sheets composed of anti-parallel loops from the twelve subunits.
ATP binds first to the top of the active site near a cation binding site, while glutamate binds near the second cation binding site at the bottom of the active site. The presence of ADP causes a conformational shift in GS that stabilizes γ-glutamyl phosphate atom. Ammonium binds strongly to GS only if the acyl-phosphate intermediate is present. Ammonium, rather than ammonia, binds to GS because the binding site is polar and exposed to solvent. In the second step, deprotonation of ammonium allows ammonia to attack the intermediate from its nearby site to form glutamine. Phosphate leaves through the top of the active site, while glutamine leave through the bottom (between two rings).
Plants have two or more isozymes of GSII, one of the isozymes is translocated into the chloroplast
.
While the three classes of GS's are clearly structurally related, the sequence similarities are not so extensive.
residue in each subunit in GS can be modified by adenylylation. Adenylyl transferase catalyzes the adenylylation and phosphorolysis reactions. Adenyl transferase activity is influenced by two regulatory proteins: PA and PD. PA reduces GS activity by attaching an AMP unit to GS. Adenylyl transferase and PD removes the AMP unit. PA and PD may be interconverted via uridylyl transferase. Adenylylated GS is less active than unadenylated GS. In the majority of gram-negative bacteria, GS can be modified by adenylylation (some cyanobacteria and green algae or exceptions).
Inhibition of GS has largely focused on amino site ligands. Other inhibitors are the result of glutamine metabolism: tryptophan, histidine, carbamoyl phosphate, glucosamine-6-phosphate, cytidine triphosphate (CTD), and adenosine monophosphate (AMP). Other inhibitors/regulators are glycine and alanine. Alanine, glycine, and serine bind to the glutamate substrate site. GDP, AMP, ADP bind to the ATP site. L-serine, L-alanine, and glycine bind to the site for L-glutamate in unadenylated GS. The four amino acids bind to the site by their common atoms, “the main chain” of amino acids. Glutamate is another product of glutamine metabolism; however, glutamate is a substrate for GS inhibiting it to act as a regulator to GS.2 Each inhibitor can reduce the activity of the enzyme; once all final glutamine metabolites are bound to GS, the activity of GS is almost completely inhibited. Many inhibitory input signals allows for fine tuning of GS by reflecting nitrogen levels in the organism.
Feedback regulation distinguishes the difference between two eukaryotic types of GS: brain and non-brain tissues. Non-brain GS responds to end-product feedback inhibition, while brain GS does not. High concentrations of glutamine-dependent metabolites should inhibit GS activity, while low concentrations should activate GS activity.
Inhibitors:
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that plays an essential role in the metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
of nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
by catalyzing the condensation of glutamate and ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
to form glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
:
Glutamate + ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
+ NH3 → Glutamine + ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
+ phosphate
Glutamine Synthetase uses ammonia produced by nitrate reduction, amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
degradation, and photorespiration
Photorespiration
Photorespiration, or "'photo-respiration'", is a process in plant metabolism by which RuBP has oxygen added to it by the enzyme , instead of carbon dioxide during normal photosynthesis. This is the beginning step of the Calvin-Benson cycle...
. The amide group of glutamate is a nitrogen source for the synthesis of glutamine pathway metabolites.
Other reactions may take place via GS. Competition between ammonium
Ammonium
The ammonium cation is a positively charged polyatomic cation with the chemical formula NH. It is formed by the protonation of ammonia...
ion and water, their binding affinities, and the concentration of ammonium ion, influences glutamine synthesis and glutamine hydrolysis. Glutamine is formed if an ammonium ion attacks the acyl-phosphate intermediate, while glutamate is remade if water attacks the intermediate. Ammonium ion binds more strongly than water to GS due to electrostatic forces between a cation and a negatively charged pocket. Another possible reaction is upon NH2OH binding to GS, rather than NH4+, yields γ-glutamylhydroxamate.
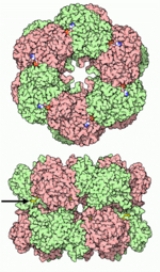
Structure
Glutamine Synthetase can be composed of 8, 10, or 12 identical subunits separated into two face-to-face rings. Bacterial GS are dodecamers with 12 active sites between each monomerMonomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
. Each active site creates a ‘bifunnel’ which is the site of three distinct substrate binding sites: nucleotide
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
, ammonium ion, and amino acid. ATP binds to the top of the bifunnel that opens to the external surface of GS. Glutamate binds at the bottom of the active site. The middle of the bifunnel contains two sites in which divalent cations bind (Mn+2 or Mg+2). One cation binding site is involved in phosphoryl transfer of ATP to glutamate, while the second stabilizes active GS and helps with the binding of glutamate.
Hydrogen bonding and hydrophobic interactions hold the two rings of GS together. Each subunit possesses a C-terminus and an N-terminus in its sequence. The C-terminus (helical thong) stabilizes the GS structure by inserting into the hydrophobic region of the subunit across in the other ring. The N-terminus is exposed to the solvent. In addition, the central channel is formed via six four-stranded β-sheets composed of anti-parallel loops from the twelve subunits.
Mechanism
GS catalyzes the ATP-dependent condensation of glutamate with ammonia to yield glutamine. The hydrolysis of ATP drives the first step of a two-part, concerted mechanism. ATP phosphorylates glutamate to form ADP and an acyl-phosphate intermediate, γ-glutamyl phosphate, which reacts with ammonia, forming glutamine and inorganic phosphate. ADP and Pi do not dissociate until ammonia binds and glutamine is released.ATP binds first to the top of the active site near a cation binding site, while glutamate binds near the second cation binding site at the bottom of the active site. The presence of ADP causes a conformational shift in GS that stabilizes γ-glutamyl phosphate atom. Ammonium binds strongly to GS only if the acyl-phosphate intermediate is present. Ammonium, rather than ammonia, binds to GS because the binding site is polar and exposed to solvent. In the second step, deprotonation of ammonium allows ammonia to attack the intermediate from its nearby site to form glutamine. Phosphate leaves through the top of the active site, while glutamine leave through the bottom (between two rings).
Biological Function
GS is present predominantly in the brain, kidneys, and liver. GS in the brain participates in the metabolic regulation of glutamate, the detoxification of brain ammonia, the assimilation of ammonia, recyclization of neurotransmitters, and termination of neurotransmitter signals. GS, in the brain, is found primarily is astrocytes. Astrocytes protect neurons against excitotoxicity by taking up excess ammonia and glutamate. In hyperammonemic environments (high levels of ammonia), astroglial swelling occurs. Different perspectives have approached the problem of astroglial swelling. One study shows that morphological changes occur that increase GS expression in glutamatergic areas or other adaptations that alleviates high levels of glutamate and ammonia. Another perspective is that astrocyte swelling is due to glutamine accumulation. To prevent increased levels of cortical glutamate and cortical water content, a study has been conducted to prevent GS activity in rats by the use of MSO.Classes
There seem to be three different classes of GS:- Class I enzymes (GSI) are specific to prokaryoteProkaryoteThe prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
s, and are oligomers of 12 identical subunitsProtein subunitIn structural biology, a protein subunit or subunit protein is a single protein molecule that assembles with other protein molecules to form a protein complex: a multimeric or oligomeric protein. Many naturally occurring proteins and enzymes are multimeric...
. The activity of GSI-type enzyme is controlled by the adenylation of a tyrosineTyrosineTyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
residue. The adenylated enzyme is inactive.
- Class II enzymes (GSII) are found in eukaryotes and in bacteria belonging to the RhizobiaceaeRhizobiaceaeThe Rhizobiaceae are a family of proteobacteria, including many species of rhizobia as well as plant parasites like Agrobacterium. Rhizobiaceae are, like all Proteobacteria, gram-negative. They are aerobic and the cells are usually rod-shaped. Many species of the Rhizobiaceae are diazotrophs, they...
, Frankiaceae, and StreptomycetaceaeStreptomycetaceaeStreptomycetaceae is a family of Actinobacteria, making up to the monotypic suborder Streptomycineae. It includes the important genus Streptomyces. This was the original source of many antibiotics, namely streptomycin. Streptomycin was the first antibiotic against tuberculosis....
families (these bacteria have also a class-I GS). GSII are decamerDecamer-See also:* Decameron * deca-, a prefix* -mer, an affix...
of identical subunits.
Plants have two or more isozymes of GSII, one of the isozymes is translocated into the chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
.
- Class III enzymes (GSIII) has, currently, only been found in Bacteroides fragilisBacteroides fragilisBacteroides fragilis is a Gram-negative bacillus bacterium species, and an obligate anaerobe of the gut.B. fragilis group is the most commonly isolated bacteriodaceae in anaerobic infections especially those that originate from the gastrointestinal flora. B. fragilis is the most prevalent organism...
and in Butyrivibrio fibrisolvens. It is a double-ringed dodecamer of identical chains. It is much larger (about 700 amino acids) than the GSI (450 to 470 amino acids) or GSII (350 to 420 amino acids) enzymes.
While the three classes of GS's are clearly structurally related, the sequence similarities are not so extensive.
Regulation & Inhibition
Reversible Covalent Modification. A tyrosineTyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
residue in each subunit in GS can be modified by adenylylation. Adenylyl transferase catalyzes the adenylylation and phosphorolysis reactions. Adenyl transferase activity is influenced by two regulatory proteins: PA and PD. PA reduces GS activity by attaching an AMP unit to GS. Adenylyl transferase and PD removes the AMP unit. PA and PD may be interconverted via uridylyl transferase. Adenylylated GS is less active than unadenylated GS. In the majority of gram-negative bacteria, GS can be modified by adenylylation (some cyanobacteria and green algae or exceptions).
Inhibition of GS has largely focused on amino site ligands. Other inhibitors are the result of glutamine metabolism: tryptophan, histidine, carbamoyl phosphate, glucosamine-6-phosphate, cytidine triphosphate (CTD), and adenosine monophosphate (AMP). Other inhibitors/regulators are glycine and alanine. Alanine, glycine, and serine bind to the glutamate substrate site. GDP, AMP, ADP bind to the ATP site. L-serine, L-alanine, and glycine bind to the site for L-glutamate in unadenylated GS. The four amino acids bind to the site by their common atoms, “the main chain” of amino acids. Glutamate is another product of glutamine metabolism; however, glutamate is a substrate for GS inhibiting it to act as a regulator to GS.2 Each inhibitor can reduce the activity of the enzyme; once all final glutamine metabolites are bound to GS, the activity of GS is almost completely inhibited. Many inhibitory input signals allows for fine tuning of GS by reflecting nitrogen levels in the organism.
Feedback regulation distinguishes the difference between two eukaryotic types of GS: brain and non-brain tissues. Non-brain GS responds to end-product feedback inhibition, while brain GS does not. High concentrations of glutamine-dependent metabolites should inhibit GS activity, while low concentrations should activate GS activity.
Inhibitors:
- Methionine Sulfoximine (MSO): MSO is an inhibitor that binds to the glutamate site. Bound to GS, MSO is phosphorylated by ATP that results in an irreversible, non-covalent inhibition of GS. The S-isomer configuration is more inhibitory. Glutamate entry is blocked into the active site by a stabilization of the flexible loop in the active site by MSO.
- Phosphinothricin(PPT, Glufosinate): Phosphinothricin is an inhibitor that binds to the glutamate site. Glufosinate is used as an herbicide. Glufosinate treated plants die due to a buildup of ammonia and a cessation of photosynthesis.
- Many synthetic inhibitors are available today.

