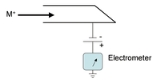
Faraday cup
Encyclopedia
A Faraday cup is a metal
(conductive) cup designed to catch charged particle
s in vacuum
. The resulting current can be measured and used to determine the number of ion
s or electron
s hitting the cup. The Faraday cup is named after Michael Faraday
who first theorized ions around 1830.
s hits the metal it gains a small net charge while the ions are neutralized. The metal can then be discharged to measure a small current equivalent to the number of impinging ions. Essentially the faraday cup is part of a circuit where ions are the charge carriers in vacuum and the faraday cup is the interface to the solid metal where electrons act as the charge carrier
s (as in most circuits). By measuring the electrical current (the number of electrons flowing through the circuit per second) in the metal part of the circuit the number of charges being carried by the ions in the vacuum part of the circuit can be determined. For a continuous beam of ions (each with a single charge)


where N is the number of ions observed in a time t (in seconds), I is the measured current (in amperes) and e is the elementary charge
(about 1.60 × 10−19 C). Thus, a measured current of one nanoamp (10−9 A) corresponds to about 6 billion ions striking the faraday cup each second.
Similarly, a Faraday cup can act as a collector for electrons in a vacuum (for instance from an electron beam). In this case electrons simply hit the metal plate/cup and a current is produced. Faraday cups are not as sensitive as electron multiplier
detectors, but are highly regarded for accuracy because of the direct relation between the measured current and number of ions.
from the surface struck by the incident charge and 2) backscattering (~180 degree scattering) of the incident particle, which causes it to leave the collecting surface, at least temporarily. Especially with electrons, it is fundamentally impossible to distinguish between a fresh new incident electron and one that has been backscattered or even a fast secondary electron.
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
(conductive) cup designed to catch charged particle
Charged particle
In physics, a charged particle is a particle with an electric charge. It may be either a subatomic particle or an ion. A collection of charged particles, or even a gas containing a proportion of charged particles, is called a plasma, which is called the fourth state of matter because its...
s in vacuum
Vacuum
In everyday usage, vacuum is a volume of space that is essentially empty of matter, such that its gaseous pressure is much less than atmospheric pressure. The word comes from the Latin term for "empty". A perfect vacuum would be one with no particles in it at all, which is impossible to achieve in...
. The resulting current can be measured and used to determine the number of ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s or electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s hitting the cup. The Faraday cup is named after Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
who first theorized ions around 1830.
How it works
When a beam or packet of ionIon
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s hits the metal it gains a small net charge while the ions are neutralized. The metal can then be discharged to measure a small current equivalent to the number of impinging ions. Essentially the faraday cup is part of a circuit where ions are the charge carriers in vacuum and the faraday cup is the interface to the solid metal where electrons act as the charge carrier
Charge carrier
In physics, a charge carrier is a free particle carrying an electric charge, especially the particles that carry electric currents in electrical conductors. Examples are electrons and ions...
s (as in most circuits). By measuring the electrical current (the number of electrons flowing through the circuit per second) in the metal part of the circuit the number of charges being carried by the ions in the vacuum part of the circuit can be determined. For a continuous beam of ions (each with a single charge)


where N is the number of ions observed in a time t (in seconds), I is the measured current (in amperes) and e is the elementary charge
Elementary charge
The elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
(about 1.60 × 10−19 C). Thus, a measured current of one nanoamp (10−9 A) corresponds to about 6 billion ions striking the faraday cup each second.
Similarly, a Faraday cup can act as a collector for electrons in a vacuum (for instance from an electron beam). In this case electrons simply hit the metal plate/cup and a current is produced. Faraday cups are not as sensitive as electron multiplier
Electron multiplier
An electron multiplier is a vacuum-tube structure that multiplies incident charges. In a process called secondary emission, a single electron can, when bombarded on secondary emissive material, induce emission of roughly 1 to 3 electrons...
detectors, but are highly regarded for accuracy because of the direct relation between the measured current and number of ions.
Error sources
The counting of charges collected per unit time is impacted by two error sources: 1) the emission of low-energy secondary electronsSecondary electrons
Secondary electrons are electrons generated as ionization products. They are called 'secondary' because they are generated by other radiation . This radiation can be in the form of ions, electrons, or photons with sufficiently high energy, i.e. exceeding the ionization potential...
from the surface struck by the incident charge and 2) backscattering (~180 degree scattering) of the incident particle, which causes it to leave the collecting surface, at least temporarily. Especially with electrons, it is fundamentally impossible to distinguish between a fresh new incident electron and one that has been backscattered or even a fast secondary electron.
See also
- Electron multiplierElectron multiplierAn electron multiplier is a vacuum-tube structure that multiplies incident charges. In a process called secondary emission, a single electron can, when bombarded on secondary emissive material, induce emission of roughly 1 to 3 electrons...
- Microchannel plate detectorMicrochannel plate detectorA micro-channel plate is a planar component used for detection of particles and impinging radiation . It is closely related to an electron multiplier, as both intensify single particles or photons by the multiplication of electrons via secondary emission...
- Daly detectorDaly detectorA Daly detector is a gas phase ion detector that consists of a metal "doorknob", a scintillator and a photomultiplier. It was named after its inventor Norman Richard Daly. Daly detectors are typically used in mass spectrometers....
- Faraday cup electrometerFaraday cup electrometerThe Faraday cup electrometer is the simplest form of an electrical aerosol instrument used in aerosol studies. It consists of an electrometer and a filter inside a Faraday cage...
- Faraday cageFaraday cageA Faraday cage or Faraday shield is an enclosure formed by conducting material or by a mesh of such material. Such an enclosure blocks out external static and non-static electric fields...
- Faraday constant

