
F-TEDA-BF4
Encyclopedia
1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) or Selectfluor® , a trademark of Air Products and Chemicals, is a reagent
in chemistry
that is used as a fluorine
donor. This compound is a derivative of the heterocycle DABCO
® . This colourless salt was first described in 1992 and has since been commercialized for use in organofluorine chemistry
.
of diazabicyclo[2.2.2]octane (DABCO® ) with dichloromethane
, followed by ion exchange
with sodium tetrafluoroborate
(replacing the chloride counterion
for the tetrafluoroborate). Finally, this salt is treated with elemental fluorine and sodium tetrafluoroborate:
. Oxidation of alcohol
s and phenols
. As applied to electrophilic iodination, Selectfluor® reagent activates I2 bond.
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
in chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
that is used as a fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
donor. This compound is a derivative of the heterocycle DABCO
DABCO
DABCO or 1,4-diazabicyclo[2.2.2]octane is a chemical compound. It is a polyurethane and Baylis-Hillman reaction catalyst, complexing ligand and Lewis base. It is used to regulate the reaction rate in Flexplay time-limited DVDs by adjusting pH. Antioxidants, like DABCO, are used to improve the...
® . This colourless salt was first described in 1992 and has since been commercialized for use in organofluorine chemistry
Organofluorine chemistry
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil- and water-repellents to pharmaceuticals, refrigerants and reagents in catalysis...
.
Preparation
Selectfluor® reagent is commercially available. It is synthesized by the alkylationAlkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
of diazabicyclo[2.2.2]octane (DABCO® ) with dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
, followed by ion exchange
Ion exchange
Ion exchange is an exchange of ions between two electrolytes or between an electrolyte solution and a complex. In most cases the term is used to denote the processes of purification, separation, and decontamination of aqueous and other ion-containing solutions with solid polymeric or mineralic 'ion...
with sodium tetrafluoroborate
Sodium tetrafluoroborate
Sodium tetrafluoroborate, also called sodium borofluoride, is a compound with formula NaBF4. It forms colorless water-soluble rhombic crystals and is soluble in water and less soluble in organic solvents....
(replacing the chloride counterion
Counterion
A counterion is the ion that accompanies an ionic species in order to maintain electric neutrality. In table salt the sodium cation is the counterion for the chlorine anion and vice versa.In a charged transition metal complex, a simple A counterion is the ion that accompanies an ionic species in...
for the tetrafluoroborate). Finally, this salt is treated with elemental fluorine and sodium tetrafluoroborate:
Applications
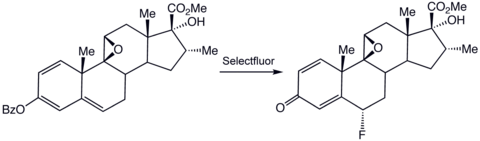
The conventional source of "electrophilic fluorine," i.e. the equivalent to the superelectrophile F+, is gaseous fluorine, which requires specialised equipment for manipulation. Selectfluor® reagent is a salt that requires only routine procedures for its use. Like F2, the salt delivers the equivalent of F+. It is mainly used in the synthesis of organofluorine compounds:
Specialized applications
Selectfluor® reagent also serves as a strong oxidant, a property that is useful in other reactions in organic chemistryOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. Oxidation of alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s and phenols
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group...
. As applied to electrophilic iodination, Selectfluor® reagent activates I2 bond.
Other References
- Lal, G. S., J. Org. Chem. 1993, 58, 2791.
- Lal, G. S., Synth. Commun. 1995, 25 (5), 725
- Banks, R. E.; Lawrence, N. J.; Popplewell, A. L., J. Chem. Soc., Chem. Commun. 1994, 343.
- Zupan, M.; Iskra, J.; Stavber, S., J. Fluorine Chem., 1995, 70, 7.
- Matthews, D.P.; Miller, S. C.; Jarvi E. T.; Sabol, J. S.; McCarthy, J. R., Tettrahedron Lett. 1993, 34 (19), 3057.
- Brunaus, M.; Dell, C. P.; Owton, W. M., J. Fluorine Chem. 1994, 201.
- McClinton, M. A. ; Sik, V., J. Chem. Soc., Perkin Trans. I, 1992, 1891.
- Hodson, H. F.; Madge, D. J.; Slawin, A. N. Z.; Widdawson, D. A.; Williams, D. J., Tetrahedron, 1994, 50 (6), 1899.
- Stavber, S.; Zupan, M., J. Chem. Soc., Chem. Commun. 1994, 149.
- Stavber, S.; Sotler, J.; Zupan, M., Tettrahedron Lett. 1994, 35 (7), 1105.

