
Lobry-de Bruyn-van Ekenstein transformation
Encyclopedia
In carbohydrate chemistry
, the Lobry–de Bruyn–van Ekenstein transformation also known as the Lobry–de Bruyn–van-Alberda–van-Ekenstein transformation is the base
or acid catalyzed
transformation of an aldose into the ketose isomer or vice versa, with a tautomeric enediol as reaction intermediate
. Ketoses may be transformed into 3-ketoses, etcetera. The enediol is also an intermediate for the epimerization of an aldose
or ketose
.
The reactions are usually base catalyzed, but can also take place under acid or neutral conditions. A typical rearrangement reaction
is that between the aldose glyceraldehyde
and the ketose dihydroxyacetone
in a chemical equilibrium
.
The Lobry–de Bruyn–van Ekenstein transformation is relevant for the industrial production of certain ketose
s and was discovered in 1885 by Cornelis Adriaan Lobry van Troostenburg de Bruyn
and Willem Alberda van Ekenstein
.
residue.
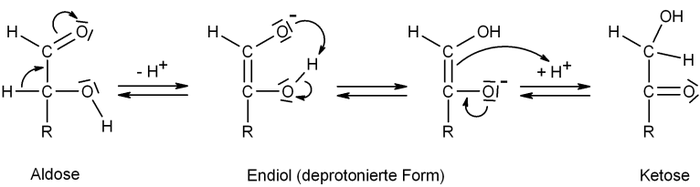
The equilibrium or the reactant to product ratio depends on concentration
, solvent
, pH
and temperature
. At equilibrium the aldose and ketose form a mixture which in the case of the glyceraldehyde and dihydroxyacetone is also called glycerose.
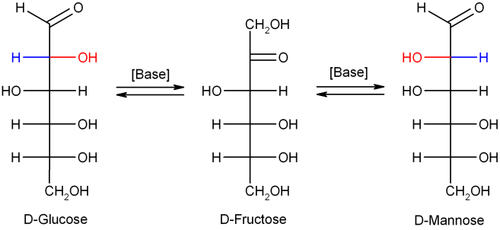
takes place is a stereocenter
. If, for example, D-glucose
(an Aldose) rearranges to D-fructose
, the ketose, the stereochemical configuration is lost in the enol
form. In the chemical reaction the enol can be protonated from two faces, resulting in the backformation of glucose or the formation of the epimer
D-mannose
. The final product is a mix of D-glucose, D-fructose and D-mannose.
Carbohydrate chemistry
Carbohydrate chemistry is a subdiscipline of chemistry primarily concerned with the synthesis, structure, and function of carbohydrate structures. Due to the general structure of carbohydrates, their synthesis is often preoccupied with the selective formation of glycosidic linkages and the...
, the Lobry–de Bruyn–van Ekenstein transformation also known as the Lobry–de Bruyn–van-Alberda–van-Ekenstein transformation is the base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
or acid catalyzed
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
transformation of an aldose into the ketose isomer or vice versa, with a tautomeric enediol as reaction intermediate
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
. Ketoses may be transformed into 3-ketoses, etcetera. The enediol is also an intermediate for the epimerization of an aldose
Aldose
An aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
or ketose
Ketose
A ketose is a sugar containing one ketone group per molecule.With 3 carbon atoms, dihydroxyacetone is the simplest of all ketoses and is the only one having no optical activity. Ketoses can isomerize into an aldose when the carbonyl group is located at the end of the molecule...
.
The reactions are usually base catalyzed, but can also take place under acid or neutral conditions. A typical rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
is that between the aldose glyceraldehyde
Glyceraldehyde
Glyceraldehyde is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism...
and the ketose dihydroxyacetone
Dihydroxyacetone
Dihydroxyacetone , or DHA, also known as glycerone, is a simple carbohydrate with formula .DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, and by the fermentation of glycerin.-Chemistry:DHA is a...
in a chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
.
The Lobry–de Bruyn–van Ekenstein transformation is relevant for the industrial production of certain ketose
Ketose
A ketose is a sugar containing one ketone group per molecule.With 3 carbon atoms, dihydroxyacetone is the simplest of all ketoses and is the only one having no optical activity. Ketoses can isomerize into an aldose when the carbonyl group is located at the end of the molecule...
s and was discovered in 1885 by Cornelis Adriaan Lobry van Troostenburg de Bruyn
Cornelis Adriaan Lobry van Troostenburg de Bruyn
Cornelis Adriaan Lobry van Troostenburg de Bruyn was a chemist from the Netherlands.-Biography:De Bruyn was born on in Leeuwarden, where his father, Nicholaas Lobry van Troostenburg de Bruyn, was a physician in practice. The boy was in due time sent to the high school of the town , and...
and Willem Alberda van Ekenstein
Willem Alberda van Ekenstein
Willem Alberda van Ekenstein was a Dutch chemist and discovered the Lobry-de Bruyn-van Ekenstein transformation together with Adriaan Lobry van Troostenburg de Bruyn....
.
Aldose-ketose transformation
The following scheme describes the interconversion between an aldose and a ketose, where R is any organicOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
residue.

The equilibrium or the reactant to product ratio depends on concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
, solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
. At equilibrium the aldose and ketose form a mixture which in the case of the glyceraldehyde and dihydroxyacetone is also called glycerose.
Epimerization
The carbon atom at which the initial deprotonationDeprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
takes place is a stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
. If, for example, D-glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
(an Aldose) rearranges to D-fructose
Fructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
, the ketose, the stereochemical configuration is lost in the enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
form. In the chemical reaction the enol can be protonated from two faces, resulting in the backformation of glucose or the formation of the epimer
Epimer
In chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
D-mannose
Mannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. Mannose is a C-2 epimer of glucose. It is not part of human metabolism, but is a component of microbial cell walls, and is therefore a target of the immune system and also of antibiotics....
. The final product is a mix of D-glucose, D-fructose and D-mannose.


