
Diffusionless transformations
Encyclopedia
A diffusionless transformation is a phase change
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
that occurs without the long-range diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of atoms but rather by some form of cooperative, homogeneous movement of many atoms that results in a change in crystal structure. These movements are small, usually less than the interatomic distances, and the atoms maintain their relative relationships. The ordered movement of large numbers of atoms lead some to refer to these as military transformations in contrast to civilian diffusion-based phase changes.
The most commonly encountered transformation of this type is the martensitic
Adolf Martens
Adolf Martens , 6 March 1850 – 24 July 1914, was a German metallurgist and the namesake of the steel structure martensite.-References:*...
transformation which, while being the best known, is actually only one subset of non-diffusional transformations. The martensitic transformation in steel represents the most economically important example of this category of phase transformations but an increasing number of alternatives, such as shape memory alloy
Shape memory alloy
A shape-memory alloy is an alloy that "remembers" its original, cold-forged shape: returning the pre-deformed shape by heating. This material is a lightweight, solid-state alternative to conventional actuators such as hydraulic, pneumatic, and motor-based systems...
s, are leaving the bounds of pure scientific interest and having significant impact on day-to-day life.
Classification and definitions
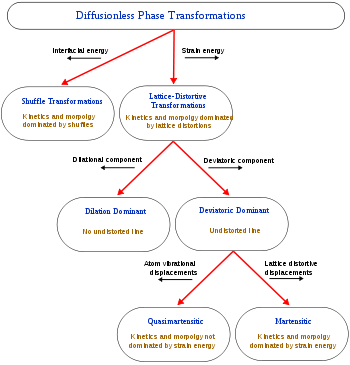
When a structural change occurs by the coordinated movement of atoms (or groups of atoms) relative to their neighbors then the change is termed displacive transformation. This covers a broad range of transformations and so further classifications have been developed [Cohen 1979].The first distinction can be drawn between transformations dominated by lattice-distortive strains and those where shuffles are of greater importance.
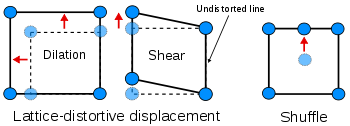
Homogeneous lattice-distortive strains, also known as Bain strains, are strains that transform one Bravais lattice into a different one. This can be represented by a strain matrix
Matrix (mathematics)
In mathematics, a matrix is a rectangular array of numbers, symbols, or expressions. The individual items in a matrix are called its elements or entries. An example of a matrix with six elements isMatrices of the same size can be added or subtracted element by element...
S which transforms one vector, y, into a new vector, x:

This is homogeneous as straight lines are transformed to new straight lines. Examples of such transformations include a cubic lattice
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
increasing in size on all three axes (dilation) or shearing
Shearing (physics)
Shearing in continuum mechanics refers to the occurrence of a shear strain, which is a deformation of a material substance in which parallel internal surfaces slide past one another. It is induced by a shear stress in the material...
into a monoclinic
Monoclinic crystal system
In crystallography, the monoclinic crystal system is one of the 7 lattice point groups. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal length, as in the orthorhombic system. They form a rectangular prism with a...
structure.
Phase transformations normally result in the creation of an interface between the transformed and parent material. The energy required to generate this new interface will depend on its nature - essentially how well the two structures fit together. An additional energy term occurs if the transformation includes a shape change since, if new phase is constrained by surrounding material, this may give rise to elastic
Elasticity (physics)
In physics, elasticity is the physical property of a material that returns to its original shape after the stress that made it deform or distort is removed. The relative amount of deformation is called the strain....
or plastic
Plastic
A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass, and may contain other substances to improve performance and/or reduce production costs...
deformation and hence a strain
Strain (materials science)
In continuum mechanics, the infinitesimal strain theory, sometimes called small deformation theory, small displacement theory, or small displacement-gradient theory, deals with infinitesimal deformations of a continuum body...
energy term. The ratio of these interfacial and strain energy terms has a notable effect on the kinetics of the transformation and the morphology of the new phase. Thus, shuffle transformations, where distortions are small, are dominated by interfacial energies and can be usefully separated from lattice-distortive transformations where the strain energy tends to have a greater effect.
A subclassification of lattice-distortive displacements can be made by considering the dilational and shear components of the distortion. In transformations dominated by the shear component it is possible to find a line in the new phase that is undistorted from the parent phase while all lines are distorted when the dilation is predominant. Shear dominated transformations can be further classified according to the magnitude of the strain energies involved compared to the innate vibrations
Atom vibrations
The atoms and ions, which are bonded with each other with considerable interatomic forces, are not motionless. Due to the consistent vibrating movements, they are permanently deviating from their equilibrium position. Elastic waves of different lengths, frequencies, and amplitudes run through...
of the atoms in the lattice and hence whether the strain energies have a notable influence on the kinetics of the transformation and the morphology of the resulting phase. If the strain energy is a significant factor then the transformations are dubbed martensitic and if it is not the transformation is referred to as quasi-martensitic.
Martensitic transformation
The difference between austeniteAustenite
Austenite, also known as gamma phase iron, is a metallic non-magnetic allotrope of iron or a solid solution of iron, with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of ; other alloys of steel have different eutectoid temperatures...
and martensite
Martensite
Martensite, named after the German metallurgist Adolf Martens , most commonly refers to a very hard form of steel crystalline structure, but it can also refer to any crystal structure that is formed by displacive transformation. It includes a class of hard minerals occurring as lath- or...
is, in some ways, quite small: while the unit cell of austenite is, on average, a perfect little cube, the transformation to martensite sees this cube distorted by interstitial carbon atoms that do not have time to diffuse out during displacive transformation, so that it is a tiny bit longer than before in one dimension and a little bit shorter in the other two. The mathematical description of the two structures is quite different, for reasons of symmetry (see external links), but the chemical bonding remains very similar. Unlike cementite
Cementite
Cementite, also known as iron carbide, is a chemical compound of iron and carbon, with the formula Fe3C . By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, brittle material, normally classified as a ceramic in its pure form, though it is more...
, which has bonding reminiscent of ceramic materials, the hardness of martensite is difficult to explain in chemical terms.
The explanation hinges on the crystal's subtle change in dimension. Even a microscopic crystallite is millions of unit cells long. Since all of these units face the same direction, distortions of even a fraction of a percent become magnified into a major mismatch between neighboring materials. The mismatch is sorted out by the creation of a myriad of crystal defects, in a process reminiscent of work hardening
Work hardening
Work hardening, also known as strain hardening or cold working, is the strengthening of a metal by plastic deformation. This strengthening occurs because of dislocation movements within the crystal structure of the material. Any material with a reasonably high melting point such as metals and...
. As in work-hardened steel, these defects prevent atoms from sliding past one another in an organized fashion, causing the material to become harder.
Shape memory alloys also have surprising mechanical properties, that were eventually explained by an analogy to martensite. Unlike the iron-carbon system, alloys in the nickel-titanium system can be chosen that make the "martensitic" phase thermodynamically
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
stable.

