
Diffusion creep
Encyclopedia
Diffusion creep refers to the deformation
of crystalline solids by the diffusion
of vacancies through their crystal lattice. Diffusion creep results in plastic deformation rather than brittle failure of the material.
Diffusion creep is more sensitive to temperature
than other deformation mechanism
s. It usually takes place at high homologous temperature
s (i.e. within about a tenth of its absolute melting
temperature). Diffusion creep is caused by the migration of crystalline defects
through the lattice of a crystal such that when a crystal is subjected to a greater degree of compression in one direction relative to another, defects migrate to the crystal faces along the direction of compression, causing a net mass transfer that shortens the crystal in the direction of maximum compression. The migration of defects is in part due to vacancies, whose migration is equal to a net mass transport in the opposite direction.
. The number of vacancies may also be influenced by the number of chemical impurities in the crystal lattice, if such impurities require the formation of vacancies to exist in the lattice.
A vacancy can move through the crystal structure when the neighbouring particle "jumps" in the vacancy, so that the vacancy moves in effect one site in the crystal lattice. Chemical bond
s need to be broken and new bonds have to be formed during the process, therefore a certain activation energy
is needed. Moving a vacancy through a crystal becomes therefore easier when the temperature
is higher.
The most stable state will be when all vacancies are evenly spread through the crystal. This principle follows from Fick's law:
In which Jx stands for the flux
("flow") of vacancies in direction x; Dx is a constant
for the material in that direction and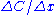 is the difference in concentration of vacancies in that direction. The law is valid for all principal directions in (x, y, z)-space, so the x in the formula can be exchanged for y or z. The result will be that they will become evenly distributed over the crystal, which will result in the highest mixing entropy
is the difference in concentration of vacancies in that direction. The law is valid for all principal directions in (x, y, z)-space, so the x in the formula can be exchanged for y or z. The result will be that they will become evenly distributed over the crystal, which will result in the highest mixing entropy
.
When a mechanical stress is applied to the crystal, new vacancies will be created at the sides perpendicular to the direction of the lowest principal stress. The vacancies will start moving in the direction of crystal planes perpendicular to the maximal stress. Current theory holds that the elastic
strain
in the neighborhood of a defect is smaller toward the axis of greatest differential compression, creating a defect chemical potential gradient
(depending upon lattice strain) within the crystal that leads to net accumulation of defects at the faces of maximum compression by diffusion. A flow of vacancies is the same as a flow of particles in the opposite direction. This means a crystalline material can deform under a differential stress
, by the flow of vacancies.
Highly mobile chemical components substituting for other species in the lattice can also cause a net differential mass transfer (i.e. segregation) of chemical species inside the crystal itself, often promoting shortening of the rheologically
more difficult substance and enhancing deformation.
, a mechanism called Coble creep
.
When a crystal deforms by diffusion creep to accommodate space problems from simultaneous grain boundary sliding (the movement of whole grains along grain boundaries) this is called granular or superplastic flow. Diffusion creep can also be simultaneous with pressure solution
. Pressure solution is, like Coble creep, a mechanism in which material moves along grain boundaries. While in Coble creep the particles move by "dry" diffusion, in pressure solution they move in solution.
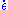 ) depends on the differential stress (σ or σD), the grain size (d) and an activation value in the form of an Arrhenius equation
) depends on the differential stress (σ or σD), the grain size (d) and an activation value in the form of an Arrhenius equation
:
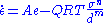
In which A is the constant of diffusion, Q the activation energy of the mechanism, R the gas constant
and T the absolute temperature (in kelvins). The exponents n and m are values for the sensitivity of the flow to stress and grain size respectively. The values of A, Q, n and m are different for each deformation mechanism. For diffusion creep, the value of n is usually around 1. The value for m can vary between 2 (Nabarro-Herring creep) and 3 (Coble creep). That means Coble creep is more sensitive to grain size of a material: materials with larger grains can deform less easily by Coble creep than materials with small grains.
Diffusion creep is a mechanism by which the volume of the crystals can increase. Larger grain sizes can be a sign that diffusion creep was more effective in a crystalline material.
Deformation (mechanics)
Deformation in continuum mechanics is the transformation of a body from a reference configuration to a current configuration. A configuration is a set containing the positions of all particles of the body...
of crystalline solids by the diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of vacancies through their crystal lattice. Diffusion creep results in plastic deformation rather than brittle failure of the material.
Diffusion creep is more sensitive to temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
than other deformation mechanism
Deformation mechanism
In structural geology, metallurgy and materials science, deformation mechanisms refer to the various mechanisms at the grain scale that are responsible for accommodating large plastic strains in rocks, metals and other materials.-Mechanisms:...
s. It usually takes place at high homologous temperature
Homologous temperature
Homologous temperature expresses the temperature of a material as a fraction of its melting point temperature using the Kelvin scale. For example, the homologous temperature of lead at room temperature is approximately 0.50 ....
s (i.e. within about a tenth of its absolute melting
Melting
Melting, or fusion, is a physical process that results in the phase change of a substance from a solid to a liquid. The internal energy of a substance is increased, typically by the application of heat or pressure, resulting in a rise of its temperature to the melting point, at which the rigid...
temperature). Diffusion creep is caused by the migration of crystalline defects
Crystallographic defect
Crystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
through the lattice of a crystal such that when a crystal is subjected to a greater degree of compression in one direction relative to another, defects migrate to the crystal faces along the direction of compression, causing a net mass transfer that shortens the crystal in the direction of maximum compression. The migration of defects is in part due to vacancies, whose migration is equal to a net mass transport in the opposite direction.
Principle
Crystalline materials are never perfect on a microscale. Some sites of atoms in the crystal lattice can be occupied by point defects, such as "alien" particles or vacancies. Vacancies can actually be thought of as chemical species themselves (or part of a compound species/component) that may then be treated using heterogeneous phase equilibriaGibbs' phase rule
Gibbs' phase rule was proposed by Josiah Willard Gibbs in the 1870s as the equalityF\;=\;C\;-\;P\;+\;2where P is the number of phases in thermodynamic equilibrium with each other and C is the number of components. Typical phases are solids, liquids and gases. A system involving one pure chemical...
. The number of vacancies may also be influenced by the number of chemical impurities in the crystal lattice, if such impurities require the formation of vacancies to exist in the lattice.
A vacancy can move through the crystal structure when the neighbouring particle "jumps" in the vacancy, so that the vacancy moves in effect one site in the crystal lattice. Chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s need to be broken and new bonds have to be formed during the process, therefore a certain activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
is needed. Moving a vacancy through a crystal becomes therefore easier when the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
is higher.
The most stable state will be when all vacancies are evenly spread through the crystal. This principle follows from Fick's law:
In which Jx stands for the flux
Flux
In the various subfields of physics, there exist two common usages of the term flux, both with rigorous mathematical frameworks.* In the study of transport phenomena , flux is defined as flow per unit area, where flow is the movement of some quantity per time...
("flow") of vacancies in direction x; Dx is a constant
Constant (mathematics)
In mathematics, a constant is a non-varying value, i.e. completely fixed or fixed in the context of use. The term usually occurs in opposition to variable In mathematics, a constant is a non-varying value, i.e. completely fixed or fixed in the context of use. The term usually occurs in opposition...
for the material in that direction and
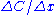 is the difference in concentration of vacancies in that direction. The law is valid for all principal directions in (x, y, z)-space, so the x in the formula can be exchanged for y or z. The result will be that they will become evenly distributed over the crystal, which will result in the highest mixing entropy
is the difference in concentration of vacancies in that direction. The law is valid for all principal directions in (x, y, z)-space, so the x in the formula can be exchanged for y or z. The result will be that they will become evenly distributed over the crystal, which will result in the highest mixing entropyEntropy of mixing
In thermodynamics the entropy of mixing is the increase in the total entropy of a compound system, when different and chemically non-reacting chemical substances or material components are mixed by removing partition between the system's initially separate volumes...
.
When a mechanical stress is applied to the crystal, new vacancies will be created at the sides perpendicular to the direction of the lowest principal stress. The vacancies will start moving in the direction of crystal planes perpendicular to the maximal stress. Current theory holds that the elastic
Elasticity (physics)
In physics, elasticity is the physical property of a material that returns to its original shape after the stress that made it deform or distort is removed. The relative amount of deformation is called the strain....
strain
Strain (materials science)
In continuum mechanics, the infinitesimal strain theory, sometimes called small deformation theory, small displacement theory, or small displacement-gradient theory, deals with infinitesimal deformations of a continuum body...
in the neighborhood of a defect is smaller toward the axis of greatest differential compression, creating a defect chemical potential gradient
Potential gradient
A potential gradient is the local space rate of change of the potential with respect to displacement.In electrostatics then, it is the local space rate of change of the electric potential:Units are volts per meter...
(depending upon lattice strain) within the crystal that leads to net accumulation of defects at the faces of maximum compression by diffusion. A flow of vacancies is the same as a flow of particles in the opposite direction. This means a crystalline material can deform under a differential stress
Differential stress
Differential stress is the difference between the greatest and the least compressive stress experienced by an object. For both the geological and civil engineering convention \sigma_1 is the greatest compressive stress and \sigma_3 is the weakest,...
, by the flow of vacancies.
Highly mobile chemical components substituting for other species in the lattice can also cause a net differential mass transfer (i.e. segregation) of chemical species inside the crystal itself, often promoting shortening of the rheologically
Rheology
Rheology is the study of the flow of matter, primarily in the liquid state, but also as 'soft solids' or solids under conditions in which they respond with plastic flow rather than deforming elastically in response to an applied force....
more difficult substance and enhancing deformation.
Types of diffusion creep
Diffusion of vacancies through a crystal can happen in a number of ways. When vacancies move through the crystal (in the material sciences often called a "grain"), this is called Nabarro-Herring creep. Another way in which vacancies can move is along the grain boundariesGrain boundary
A grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are defects in the crystal structure, and tend to decrease the electrical and thermal conductivity of the material...
, a mechanism called Coble creep
Coble creep
Coble creep, a form of diffusion creep, is a mechanism for deformation of crystalline solids. Coble creep occurs through the diffusion of atoms in a material along the grain boundaries, which produces a net flow of material and a sliding of the grain boundaries.Coble creep is named after Robert L...
.
When a crystal deforms by diffusion creep to accommodate space problems from simultaneous grain boundary sliding (the movement of whole grains along grain boundaries) this is called granular or superplastic flow. Diffusion creep can also be simultaneous with pressure solution
Pressure solution
In structural geology and diagenesis, pressure solution or pressure dissolution is a deformation mechanism that involves the dissolution of minerals at grain-to-grain contacts into an aqueous pore fluid in areas of relatively high stress and either deposition in regions of relatively low stress...
. Pressure solution is, like Coble creep, a mechanism in which material moves along grain boundaries. While in Coble creep the particles move by "dry" diffusion, in pressure solution they move in solution.
Flow laws
Each plastic deformation of a material can be described by a formula in which the strain rate (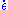 ) depends on the differential stress (σ or σD), the grain size (d) and an activation value in the form of an Arrhenius equation
) depends on the differential stress (σ or σD), the grain size (d) and an activation value in the form of an Arrhenius equationArrhenius equation
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
:

In which A is the constant of diffusion, Q the activation energy of the mechanism, R the gas constant
Gas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
and T the absolute temperature (in kelvins). The exponents n and m are values for the sensitivity of the flow to stress and grain size respectively. The values of A, Q, n and m are different for each deformation mechanism. For diffusion creep, the value of n is usually around 1. The value for m can vary between 2 (Nabarro-Herring creep) and 3 (Coble creep). That means Coble creep is more sensitive to grain size of a material: materials with larger grains can deform less easily by Coble creep than materials with small grains.
Traces of diffusion creep
It is difficult to find clear microscale evidence for diffusion creep in a crystalline material, since little structures have been identified as definite proof. A material that was deformed by diffusion creep can have flattened grains (grains with a so called shape-preferred orientation or SPO). Equidimensional grains with no lattice-preferred orientation (or LPO) can be an indication for superplastic flow. In materials that were deformed under very high temperatures, lobate grain boundaries may be taken as evidence for diffusion creep.Diffusion creep is a mechanism by which the volume of the crystals can increase. Larger grain sizes can be a sign that diffusion creep was more effective in a crystalline material.
See also
- creep (deformation)Creep (deformation)In materials science, creep is the tendency of a solid material to slowly move or deform permanently under the influence of stresses. It occurs as a result of long term exposure to high levels of stress that are below the yield strength of the material....
- deformation (engineering)
- diffusionDiffusionMolecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
- dislocation creepDislocation creepDislocation creep is a deformation mechanism in crystalline materials. Dislocation creep involves the movement of dislocations through the crystal lattice of the material. It causes plastic deformation of the individual crystals and in the end the material itself.Dislocation creep is highly...
- material sciences
Literature
- Gower, R.J.W. & Simpson, C.; 1992: Phase boundary mobility in naturally deformed, high-grade quartzofeldspatic rocks: evidence for diffusion creep, Journal of Structural Geology 14, p. 301-314.
- Passchier, C.W. & Trouw, R.A.J., 1998: Microtectonics, Springer, ISBN 3-540-58713-6
- Twiss, R.J. & Moores, E.M., 2000 (6th edition): Structural Geology, W.H. Freeman & co, ISBN 0-7167-2252-6


