Di-tungsten tetra(hpp)
Encyclopedia
Di-tungsten tetra is the name of the coordination compound with the formula W2(hpp)4. This material consists of a pair of tungsten
centers linked by the conjugate base of four hexahydropyrimidopyrimidine (hpp) ligand
s. It adopts a structure sometimes called a "paddlewheel" or "Chinese lantern" structure
(paddlewheel compound), the prototype being copper(II) acetate
.
The molecule is of theoretical interest because it has the lowest ionization energy
(3.51 eV) of all stable chemical element
s or chemical compound
s as of the year 2005. This value is even lower than of caesium
with 3.89 eV (or 375 kJ/mol) located at the extreme left lower corner of the periodic table
(although francium
is at a lower position in the periodic table compared to caesium, it has a higher ionization energy and is radioactive) or known metallocene
reducing agent
s such as decamethylcobaltocene
with 4.71 eV.

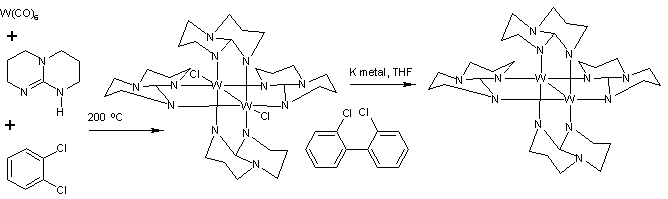
with 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine (Hhpp) in o-dichlorobenzene at 200 °C:
The reaction gives W2(hpp)4Cl2. Dichlorobenzene provides the chlorine atoms and is itself reductively coupled to dichlorobiphenyl
. The bond order
between the tungsten centers in W2(hpp)4Cl2 is three.
This dichloride is further reduced by potassium
metal to W2(hpp)4. This species has a quadruple bond
between the two tungsten centers. Related quadruply bonded complexes include and
. Because of its low ionization energy, W2(hpp)4 is easily oxidized back to the dichloride by dichloromethane
. It is readily oxidized to the corresponding cation with the oxidants fullerene
and with tetracyanoquinodimethane.
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
centers linked by the conjugate base of four hexahydropyrimidopyrimidine (hpp) ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s. It adopts a structure sometimes called a "paddlewheel" or "Chinese lantern" structure
Chinese lantern structure
In chemistry, the Chinese lantern structure is a coordination complex where two metal atoms are bridged by four bidentate ligands. An example is copper acetate dihydrate. The name reflects a resemblance between ball-and-stick models of the structure and a Chinese paper lantern....
(paddlewheel compound), the prototype being copper(II) acetate
Copper(II) acetate
Copper acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu2 where OAc- is acetate . The hydrated derivative, which contains one molecule of water for each Cu atom, is available commercially. Anhydrous Cu2 is a dark green crystalline solid, whereas Cu22 is...
.
The molecule is of theoretical interest because it has the lowest ionization energy
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
(3.51 eV) of all stable chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
s or chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s as of the year 2005. This value is even lower than of caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
with 3.89 eV (or 375 kJ/mol) located at the extreme left lower corner of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
(although francium
Francium
Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
is at a lower position in the periodic table compared to caesium, it has a higher ionization energy and is radioactive) or known metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
s such as decamethylcobaltocene
Cobaltocene
Cobaltocene, known also as biscobalt or even "bis Cp cobalt", is an organocobalt compound with the formula Co2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene...
with 4.71 eV.

Preparation
This coordination compound is prepared by the reaction of tungsten hexacarbonylTungsten hexacarbonyl
Tungsten hexacarbonyl is the chemical compound with the formula W6. This complex gave rise to the first example of a dihydrogen complex....
with 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine (Hhpp) in o-dichlorobenzene at 200 °C:
The reaction gives W2(hpp)4Cl2. Dichlorobenzene provides the chlorine atoms and is itself reductively coupled to dichlorobiphenyl
Biphenyl
Biphenyl is an organic compound that forms colorless crystals. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula 2...
. The bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
between the tungsten centers in W2(hpp)4Cl2 is three.
This dichloride is further reduced by potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
metal to W2(hpp)4. This species has a quadruple bond
Quadruple bond
A quadruple bond is a type of chemical bond between two atoms involving eight electrons. This bond is an extension of the more familiar types double bonds and triple bonds. Stable quadruple bonds are most common among the middle members transition metal elements such rhenium, tungsten, molybdenum...
between the two tungsten centers. Related quadruply bonded complexes include and
Potassium octachlorodimolybdate
Potassium octachlorodimolybdate is the inorganic compound with the formula K4Mo2Cl8. This red-coloured salt consists of potassium cations and the octachlorodimolybdate anion, Mo2Cl84−. The anion is of historic interest because it was one of the earliest illustrations of a quadruple bonding...
. Because of its low ionization energy, W2(hpp)4 is easily oxidized back to the dichloride by dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
. It is readily oxidized to the corresponding cation with the oxidants fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
and with tetracyanoquinodimethane.