D electron count
Encyclopedia
The d electron count is a chemistry
formalism used to describe the electron configuration
of the valence electron
s of a transition metal
center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes; ligand field theory
which is an application of molecular orbital theory
to transition metals and crystal field theory
which has roots in VSEPR theory
.
for transition metals predicted by the simple Aufbau principle
and Madelung's rule has serious conflicts with experimental observations for transition metal
centers under most ambient conditions. Under most conditions all of the valence electrons of a transition metal center are located in d orbitals while the standard model of electron configuration
would predict some of them to be in the pertinent s orbital.
The valence of a transition metal center can be described by standard quantum numbers. The Aufbau principle and Madelung's rule would predict for period n that the ns orbitals fill prior to the (n-1)d orbitals. For example the 4s fills before the 3d in period 4. In general chemistry textbooks, a few exceptions are acknowledged with only one electron in the ns orbital in favor of completing a half or whole d shell. The usual explanation is that "half-filled or completely filled subshells are particularly stable arrangements of electrons". An
example is chromium whose electron configuration is [Ar] 4s1 3d5 with a half-filled d subshell, although Madelung's rule would predict 4s2 3d4. Similarly copper is [Ar] 4s1 3d10 with a full d subshell, and not [Ar] 4s2 3d9.
Matters are further complicated when metal centers are oxidized. Since the (n-1)d shell is predicted to have higher energy than the ns shell, it might be expected that electrons would be removed from the (n-1)d shell first. Experimentally it has been observed that not only are the ns electrons removed first, even for unionized complexes all of the valence electrons are located in the (n-1)d orbitals.
There are various hand waving arguments for this phenomenon including that "the ns electrons are farther away from the nuclei and thus ionized first" while ignoring results based on neutral complexes. This poor explanation avoids the basic problems with the standard electron configuration model. The standard electron configuration model assumes a hydrogen-like atom
removed from all other atoms. This assumption is only truly relevant for esoteric situations. It is far more common for metal centers to have bonds to other atoms through metallic bonds or covalent bonds. These bonds drastically change the energies of the orbitals for which electron configurations are predicted. Thus for coordination complexes the standard electron configuration formalism is meaningless and the d electron count formalism is a suitable substitute.
.png) Crystal field theory describes a number of physical phenomena well but does not describe bonding nor offer an explanation for why ns electrons are ionized before (n-1)d electrons. The more recent ligand field theory offers an easy to understand explanation the models phenomenon relatively well.
Crystal field theory describes a number of physical phenomena well but does not describe bonding nor offer an explanation for why ns electrons are ionized before (n-1)d electrons. The more recent ligand field theory offers an easy to understand explanation the models phenomenon relatively well.
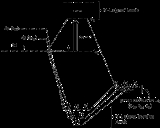
According to the model present by ligand field theory, the ns orbital is involved in bonding to the ligands and forms a strongly bonding orbital which has predominantly ligand character and the correspondingly strong anti-bonding orbital which is unfilled and usually well above the lowest unoccupied molecular orbital (LUMO). Since the orbitals resulting form the ns orbital are either buried in bonding or elevated well above the valence the ns orbitals are not relevant to the describing the valence. Depending on the geometry of the final complex either all three of the np orbitals or a portions of them are involved in bonding similar to the ns orbitals. The np orbitals if any that remain non-bonding still exceed the valence of the complex. That leaves the (n-1)d orbitals to be involved in some portion of the bonding and in the process also describe the metal complexes valence electrons. The final description of the valence is highly dependent on the complexes geometry. The complexes geometry is in turn highly dependent on the d electron count and character of the associated ligands.
For example in the MO diagram provided for the [Ti(H2O)6]3+ the ns orbital (which is placed above (n-1)d in the representation of atomic orbitals (AO)) is used in a linear combination with the ligand orbitals forming a very stable bonding orbital with significant ligand character as well as an unoccupied high energy anti-bonding orbital which is not shown. In this situation the complex geometry is octahedral
which means the two of the d orbitals have the proper geometry to be involved in bonding. The other three d orbitals in the basic model do not have significant interactions with the ligands and remain three degenerate non-bonding orbitals. The two orbitals that are involved in bonding form a linear combination with two ligand orbitals with the proper symmetry. This results in two filled bonding orbitals and two orbitals which are usually the lowest unoccupied molecular orbitals (LUMO) or the highest partially filled molecular orbitals a variation on the high occupied molecular orbitals (HOMO).
describing gradations of possible ligand field environments a metal center could experience in an octahedral
geometry. The Tanabe-Sugano diagram with a small amount of information accurately predicts absorptions in the UV and visible electromagnetic spectrum
resulting from d to d orbital electron transitions. It is these d-d transitions, ligand to metal charge transfers (LMCT), or metal to ligand charge transfers (MLCT) that generally give metals complexes their vibrant colors.
however it is possible for d0 complexes to accommodate many electron pairs (bonds/coordination number) since their d orbitals are empty and well away from the 18-electron
ceiling. Often colorless due to the lack of d to d transitions.
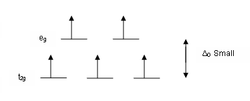
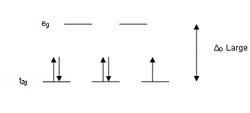
complexes in both high spin and low spin.
while low-spin d8 complexes are generally 16 electron square planar complexes. For first row transition metal complexes such as Ni2+ and Cu+ also form five coordinate 18 electron species which vary from square pyramidal to trigonal bipyramidal.
complexes limited to form 4 additional bonds (8 additional electrons) by the 18-electron
ceiling. Often colorless due to the lack of d to d transitions.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
formalism used to describe the electron configuration
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
of the valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s of a transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes; ligand field theory
Ligand field theory
Ligand field theory describes the bonding, orbital arrangement, and other characteristics of coordination complexes. It represents an application of molecular orbital theory to transition metal complexes. A transition metal ion has nine valence atomic orbitals, five d, one s, and three p orbitals...
which is an application of molecular orbital theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
to transition metals and crystal field theory
Crystal field theory
Crystal field theory is a model that describes the electronic structure of transition metal compounds, all of which can be considered coordination complexes. CFT successfully accounts for some magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes,...
which has roots in VSEPR theory
VSEPR theory
Valence shell electron pair repulsion theory is a model in chemistry used to predict the shape of individual molecules based upon the extent of electron-pair electrostatic repulsion. It is also named Gillespie–Nyholm theory after its two main developers...
.
Standard electron configuration perspective
The electron configurationElectron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
for transition metals predicted by the simple Aufbau principle
Aufbau principle
The Aufbau principle is used to determine the electron configuration of an atom, molecule or ion. The principle postulates a hypothetical process in which an atom is "built up" by progressively adding electrons...
and Madelung's rule has serious conflicts with experimental observations for transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
centers under most ambient conditions. Under most conditions all of the valence electrons of a transition metal center are located in d orbitals while the standard model of electron configuration
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
would predict some of them to be in the pertinent s orbital.
The valence of a transition metal center can be described by standard quantum numbers. The Aufbau principle and Madelung's rule would predict for period n that the ns orbitals fill prior to the (n-1)d orbitals. For example the 4s fills before the 3d in period 4. In general chemistry textbooks, a few exceptions are acknowledged with only one electron in the ns orbital in favor of completing a half or whole d shell. The usual explanation is that "half-filled or completely filled subshells are particularly stable arrangements of electrons". An
example is chromium whose electron configuration is [Ar] 4s1 3d5 with a half-filled d subshell, although Madelung's rule would predict 4s2 3d4. Similarly copper is [Ar] 4s1 3d10 with a full d subshell, and not [Ar] 4s2 3d9.
Matters are further complicated when metal centers are oxidized. Since the (n-1)d shell is predicted to have higher energy than the ns shell, it might be expected that electrons would be removed from the (n-1)d shell first. Experimentally it has been observed that not only are the ns electrons removed first, even for unionized complexes all of the valence electrons are located in the (n-1)d orbitals.
There are various hand waving arguments for this phenomenon including that "the ns electrons are farther away from the nuclei and thus ionized first" while ignoring results based on neutral complexes. This poor explanation avoids the basic problems with the standard electron configuration model. The standard electron configuration model assumes a hydrogen-like atom
Hydrogen-like atom
A hydrogen-like ion is any atomic nucleus with one electron and thus is isoelectronic with hydrogen. Except for the hydrogen atom itself , these ions carry the positive charge e, where Z is the atomic number of the atom. Examples of hydrogen-like ions are He+, Li2+, Be3+ and B4+...
removed from all other atoms. This assumption is only truly relevant for esoteric situations. It is far more common for metal centers to have bonds to other atoms through metallic bonds or covalent bonds. These bonds drastically change the energies of the orbitals for which electron configurations are predicted. Thus for coordination complexes the standard electron configuration formalism is meaningless and the d electron count formalism is a suitable substitute.
Ligand field perspective
.png)
According to the model present by ligand field theory, the ns orbital is involved in bonding to the ligands and forms a strongly bonding orbital which has predominantly ligand character and the correspondingly strong anti-bonding orbital which is unfilled and usually well above the lowest unoccupied molecular orbital (LUMO). Since the orbitals resulting form the ns orbital are either buried in bonding or elevated well above the valence the ns orbitals are not relevant to the describing the valence. Depending on the geometry of the final complex either all three of the np orbitals or a portions of them are involved in bonding similar to the ns orbitals. The np orbitals if any that remain non-bonding still exceed the valence of the complex. That leaves the (n-1)d orbitals to be involved in some portion of the bonding and in the process also describe the metal complexes valence electrons. The final description of the valence is highly dependent on the complexes geometry. The complexes geometry is in turn highly dependent on the d electron count and character of the associated ligands.
For example in the MO diagram provided for the [Ti(H2O)6]3+ the ns orbital (which is placed above (n-1)d in the representation of atomic orbitals (AO)) is used in a linear combination with the ligand orbitals forming a very stable bonding orbital with significant ligand character as well as an unoccupied high energy anti-bonding orbital which is not shown. In this situation the complex geometry is octahedral
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
which means the two of the d orbitals have the proper geometry to be involved in bonding. The other three d orbitals in the basic model do not have significant interactions with the ligands and remain three degenerate non-bonding orbitals. The two orbitals that are involved in bonding form a linear combination with two ligand orbitals with the proper symmetry. This results in two filled bonding orbitals and two orbitals which are usually the lowest unoccupied molecular orbitals (LUMO) or the highest partially filled molecular orbitals a variation on the high occupied molecular orbitals (HOMO).
Tanabe-Sugano diagram
Each of the ten possible d electron counts has an associated Tanabe-Sugano diagramTanabe-Sugano diagram
Tanabe-Sugano diagrams are used in coordination chemistry to predict absorptions in the UV and visible electromagnetic spectrum of coordination compounds. The results from a Tanabe-Sugano diagram analysis of a metal complex can also be compared to experimental spectroscopic data...
describing gradations of possible ligand field environments a metal center could experience in an octahedral
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
geometry. The Tanabe-Sugano diagram with a small amount of information accurately predicts absorptions in the UV and visible electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
resulting from d to d orbital electron transitions. It is these d-d transitions, ligand to metal charge transfers (LMCT), or metal to ligand charge transfers (MLCT) that generally give metals complexes their vibrant colors.
Limitation
It is important to remember that the d electron count is a formalism and describes some complexes better than others. Often it is difficult or impossible to assign electrons and charge to the metal center or a ligand. For a high oxidation state metal center with a 4+ charge or greater it is understood that the true charge separation is much smaller. But referring to the formal oxidation state and d electron count can still be useful when trying to understand the chemistry.Possible d electron counts
There are many examples of every possible d electron configuration. What follows is a short description of common geometries and characteristics of each possible d electron count and representative examples.d0
Commonly tetrahedralTetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
however it is possible for d0 complexes to accommodate many electron pairs (bonds/coordination number) since their d orbitals are empty and well away from the 18-electron
18-Electron rule
The 18-electron rule is a rule of thumb used primarily for predicting formulas for stable metal complexes. The rule rests on the fact that valence shells of a transition metal consists of nine valence orbitals, which collectively can accommodate 18 electrons either as nonbinding electron pairs or...
ceiling. Often colorless due to the lack of d to d transitions.
- Examples: Titanium tetrachlorideTitanium tetrachlorideTitanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
, Titanocene dichlorideTitanocene dichlorideTitanocene dichloride is the organotitanium compound with the formula 2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air...
, Schwartz's reagentSchwartz's ReagentSchwartz's reagent is the common name for the chemical compound with the formula 2ZrHCl, sometimes described zirconocene hydrochloride or zirconocene chloride hydride and is named after Jeffrey Schwartz, who is currently a professor in Chemistry at Princeton University...
.
d1
- Examples: Molybdenum(V) chlorideMolybdenum(V) chlorideMolybdenum chloride is the inorganic compound with the formula [MoCl5]2. This dark volatile solid is an important starting reagent in the preparation of molybdenum compounds. In the solid state molybdenum pentachloride exists as a dimer with the formula Mo2Cl10, with a structure similar to that...
, Vanadyl acetylacetonateVanadyl acetylacetonateVanadyl acetylacetonate is the chemical compound with the formula VO2. This blue-green coordination complex consists of the vanadyl group, VO2+, bound to two acetylacetonate anions, acac−. Like other charge-neutral acetylacetonates, this complex is soluble in organic solvents.-Synthesis:The...
, Vanadocene dichlorideVanadocene dichlorideVanadocene dichloride, dichloro bisvanadium is 2VCl2 . It is a structural analoque of titanocene dichloride but with vanadium instead of titanium....
, Vanadium tetrachlorideVanadium tetrachlorideVanadium tetrachloride is the inorganic compound with the formula VCl4. This bright red liquid is a useful reagent for the preparation of other vanadium compounds.-Synthesis, bonding, basic properties:...
.
d4
- Octahedral high-spin: 4 unpaired electrons, paramagentic, substitutionally labile.
- Octahedral low-spin: 2 unpaired electrons, paramagentic, substitutionally inert.
d5


- Octahedral high-spin: 5 unpaired electrons, paramagentic, substitutionally labile.
- Octahedral low-spin: 1 unpaired electron, paramagentic, substitutionally inert.
- Examples: Potassium ferrioxalatePotassium ferrioxalatePotassium ferrioxalate, also known as potassium oxalatoferrate, is a chemical compound with the formula K3[Fe3], where iron is in the +3 oxidation state. It is an octahedral transition metal complex in which three bidentate oxalate ions are bound to an iron center. Potassium acts as a counterion,...
, Vanadium carbonylVanadium carbonylVanadium carbonyl, also known as vanadium hexacarbonyl, is the inorganic compound with the formula V6. This highly reactive species is noteworthy from theoretical and scholarly perspectives. It is a rare isolable homoleptic metal carbonyl that is paramagnetic...
.
d6
Commonly octahedralOctahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
complexes in both high spin and low spin.
- Octahedral high-spin: 4 unpaired electrons, paramagentic, substitutionally labile.
- Octahedral low-spin: no unpaired electrons, diamagnetic, substitutionally inert.
- Examples: Cobalt(III) hexammine chlorideCobalt(III) hexammine chlorideHexamminecobalt chloride is the chemical compound with the formula [Co6]Cl3. This coordination compound is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. This salt consists of [Co6]3+ trications with three Cl− anions...
, Sodium cobaltinitriteSodium cobaltinitriteSodium cobaltinitrite is a coordination compound with the formula Na3Co6. The anion of this yellow-coloured salt consists of a cobalt center bound to six nitrito ligands. It is used as a qualitative test for potassium and ammonium ions . Although the sodium salt is soluble in water, those of...
, Molybdenum hexacarbonylMolybdenum hexacarbonylMolybdenum hexacarbonyl is the chemical compound with the formula Mo6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.-Structure and properties:Mo6 adopts an octahedral geometry consisting...
, FerroceneFerroceneFerrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
, FerroinFerroinFerroin is the chemical compound with the formula [Fe3]SO4, where o-phen is an abbreviation for 1,10-phenanthroline, a bidentate ligand. The term "ferroin" is used loosely and includes salts of other anions such as chloride.-Redox indicator:...
, Chromium carbonylChromium carbonylChromium carbonyl, also known as chromium hexacarbonyl, is the chemical compound with the formula Cr6. At room temperature the solid is stable to air, although it does have a high vapor pressure and sublimes readily. Cr6 is zerovalent, meaning that Cr has a formal charge of zero, and it is called...
.
d7
- Octahedral high spin: 3 unpaired electrons, paramagentic, substitutionally labile.
- Octahedral low spin:1 unpaired electron, paramagentic, substitutionally labile.
- Examples: CobaltoceneCobaltoceneCobaltocene, known also as biscobalt or even "bis Cp cobalt", is an organocobalt compound with the formula Co2. It is a dark purple solid that sublimes readily slightly above room temperature. Cobaltocene was discovered shortly after ferrocene, the first metallocene...
.
d8
Complexes which are d8 high-spin are usually 18 electron octahedralOctahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
while low-spin d8 complexes are generally 16 electron square planar complexes. For first row transition metal complexes such as Ni2+ and Cu+ also form five coordinate 18 electron species which vary from square pyramidal to trigonal bipyramidal.
- Octahedral high spin: 2 unpaired electrons, paramagentic, substitutionally labile.
- Square planar low spin: no unpaired electrons, diamagnetic, substitutionally inert.
- Examples: CisplatinCisplatinCisplatin, cisplatinum, or cis-diamminedichloroplatinum is a chemotherapy drug. It is used to treat various types of cancers, including sarcomas, some carcinomas , lymphomas, and germ cell tumors...
, NickeloceneNickeloceneNickelocene is the organonickel compound with the formula Ni2. Also known as bisnickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it yet has no practical applications....
, Dichlorobis(ethylenediamine)nickel(II), Iron pentacarbonylIron pentacarbonylIron pentacarbonyl, also known as iron carbonyl, is the compound with formula 5. Under standard conditions Fe5 is a free-flowing, straw-colored liquid with a pungent odour. This compound is a common precursor to diverse iron compounds, including many that are useful in organic synthesis. Fe5 is...
, Zeise's saltZeise's saltZeise's salt, potassium trichloroplatinate, is the chemical compound with the formula KPtCl3]·H2O. The anion of this air-stable, yellow, coordination complex contains an η2-ethylene ligand. The anion features a platinum atom with a square planar geometry.-Preparation:This compound is commercially...
, Vaska's complexVaska's complexVaska's complex is the trivial name for the chemical compound trans-chlorocarbonylbisiridium, which has the formula IrCl[P3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide, and a...
, Wilkinson's catalystWilkinson's catalystWilkinson's catalyst is the common name for chlorotrisrhodium, a coordination compound with the formula RhCl3 . It is named after the late organometallic chemist and 1973 Nobel Laureate, Sir Geoffrey Wilkinson who popularized its use.-Structure and basic properties:The compound is a square planar,...
.
d9
Stable complexes with this electron count is more common for first row (period four) transition metals center than it is for complexes based around second or third row transition metals centers. These include both four coordinate 17 electron species and five coordinate 19 electrons species.- Examples: Schweizer's reagentSchweizer's reagentSchweizer's reagent is the chemical complex tetraamminediaquacopper dihydroxide, [Cu42]2. It is prepared by precipitating copper hydroxide from an aqueous solution of copper sulfate using sodium hydroxide or ammonia, then dissolving the precipitate in a solution of ammonia.When the entire amount of...
.
d10
Often tetrahedralTetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
complexes limited to form 4 additional bonds (8 additional electrons) by the 18-electron
18-Electron rule
The 18-electron rule is a rule of thumb used primarily for predicting formulas for stable metal complexes. The rule rests on the fact that valence shells of a transition metal consists of nine valence orbitals, which collectively can accommodate 18 electrons either as nonbinding electron pairs or...
ceiling. Often colorless due to the lack of d to d transitions.
- Examples: Tetrakis(triphenylphosphine)palladium(0)Tetrakis(triphenylphosphine)palladium(0)Tetrakispalladium is the chemical compound Pd[P3]4, often abbreviated Pd4, or even PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.-Structure and properties:...
, Nickel carbonylNickel carbonylNickel carbonyl is the organonickel compound with the formula Ni4. This pale-yellow liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for the purification of nickel and a reagent in organometallic chemistry...
.

