Sodium cobaltinitrite
Encyclopedia
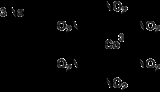
Sodium cobaltinitrite is a coordination compound with the formula Na3Co(NO2)6. The anion of this yellow-coloured salt consists of a cobalt(III) center bound to six nitrito ligands. It is used as a qualitative test for potassium
and ammonium
ions (provided that certain other cations are absent). Although the sodium salt is soluble in water, those of potassium and ammonium are insoluble. The potassium and ammonium salts are precipitated as yellow solids.
Sodium cobaltinitrite also forms the basis of a quantitative determination of potassium. However under the recommended reaction conditions the insoluble double salt, K2Na[Co(NO2)6].H2O is precipitated and weighed. Also thallium(I) can be determined as the precipitate is Tl3[Co(NO2)6].
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
and ammonium
Ammonium
The ammonium cation is a positively charged polyatomic cation with the chemical formula NH. It is formed by the protonation of ammonia...
ions (provided that certain other cations are absent). Although the sodium salt is soluble in water, those of potassium and ammonium are insoluble. The potassium and ammonium salts are precipitated as yellow solids.
Sodium cobaltinitrite also forms the basis of a quantitative determination of potassium. However under the recommended reaction conditions the insoluble double salt, K2Na[Co(NO2)6].H2O is precipitated and weighed. Also thallium(I) can be determined as the precipitate is Tl3[Co(NO2)6].

