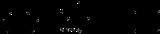
Corey-Winter olefin synthesis
Encyclopedia
The Corey-Winter olefin synthesis is a series of chemical reaction
s for converting 1,2-diol
s into olefins. It is named for the American chemist and Nobelist Elias James Corey
and the American-Estonian chemist Roland Arthur Edwin Winter.
Often, thiocarbonyldiimidazole is used instead of thiophosgene
as shown above.
involves the formation of a cyclic thio-carbonate from the diol and thiophosgene
. The second step involves treatment with trimethyl phosphite
, which attacks the sulfur
atom, producing S=P(OMe)3 (driven by the formation of a strong P=S double bond
) and leaving a carbene
. This carbene collapses with loss of carbon dioxide
to give the olefin.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s for converting 1,2-diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
s into olefins. It is named for the American chemist and Nobelist Elias James Corey
Elias James Corey
Elias James Corey is an American organic chemist. In 1990 he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis...
and the American-Estonian chemist Roland Arthur Edwin Winter.
Often, thiocarbonyldiimidazole is used instead of thiophosgene
Thiophosgene
Thiophosgene is a red liquid with the formula CSCl2. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.-Synthesis of CSCl2:...
as shown above.
Mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
involves the formation of a cyclic thio-carbonate from the diol and thiophosgene
Thiophosgene
Thiophosgene is a red liquid with the formula CSCl2. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.-Synthesis of CSCl2:...
. The second step involves treatment with trimethyl phosphite
Trimethyl phosphite
Trimethylphosphite is an organophosphorus compound with the formula P3, often abbreviated P3. This colorless liquid is used as a ligand in organometallic chemistry and as a reagent in organic synthesis...
, which attacks the sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
atom, producing S=P(OMe)3 (driven by the formation of a strong P=S double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
) and leaving a carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
. This carbene collapses with loss of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
to give the olefin.

