Trimethyl phosphite
Encyclopedia
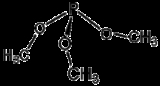
Trimethylphosphite is an organophosphorus compound with the formula
P(OCH3)3, often abbreviated P(OMe)3. This colorless liquid is used as a ligand
in organometallic chemistry
and as a reagent in organic synthesis
. The molecule features a pyramidal phosphorus(III) center bound to three methoxide
groups.
:
It is susceptible to oxidation to trimethyl phosphate
.
As a ligand, trimethylphosphite has a smaller cone angle and better acceptor properties relative to trimethylphosphine
. A representative derivative is the colorless, tetrahedral complex Ni(P(OMe)3)4 (m.p.
108 °C). The tridentate ligand called the Klaui ligand is derived from trimethylphosphite. The formation of this ligand illustrates the susceptibility of trimethylphosphite (and metal complexes thereof) to the Arbuzov reaction.
Trimethylphosphite is also used as a mild desulfurization reagent in organic synthesis
, for example in the preparation of derivatives of tetrathiafulvalene
.
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
P(OCH3)3, often abbreviated P(OMe)3. This colorless liquid is used as a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
and as a reagent in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. The molecule features a pyramidal phosphorus(III) center bound to three methoxide
Methoxide
Methoxides are organic salts and the simplest alkoxides. Sodium methoxide and potassium hydroxide have widespread use, though other variants such as lithium hydroxide, rubidium methoxide, caesium methoxide, and francium methoxide exist as well.- Methoxide ion :In organic chemistry, the methoxide...
groups.
Synthesis and reactions
Although commercially available, trimethylphosphite is prepared from phosphorus trichloridePhosphorus trichloride
Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having chemical formula PCl3. Its shape is trigonal pyramidal. It is the most important of the three phosphorus chlorides. It is an important industrial chemical, being used for the manufacture of organophosphorus compounds...
:
- PCl3 + 3 CH3OH → P(OCH3)3 + 3 HCl
It is susceptible to oxidation to trimethyl phosphate
Trimethyl phosphate
Trimethyl phosphate is the trimethyl ester of phosphoric acid. It is a colourless, nonvolatile liquid. It has some specialized uses in the production of other compounds.-Production:...
.
As a ligand, trimethylphosphite has a smaller cone angle and better acceptor properties relative to trimethylphosphine
Trimethylphosphine
Trimethylphosphine is the organophosphorus compound with the formula P3, commonly abbreviated PMe3. This colorless liquid has a strongly unpleasant odour, which is characteristic of alkylphosphines. It is a pyramidal molecule with C3v symmetry, similar to ammonia and phosphine . As a ligand, its...
. A representative derivative is the colorless, tetrahedral complex Ni(P(OMe)3)4 (m.p.
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
108 °C). The tridentate ligand called the Klaui ligand is derived from trimethylphosphite. The formation of this ligand illustrates the susceptibility of trimethylphosphite (and metal complexes thereof) to the Arbuzov reaction.
Trimethylphosphite is also used as a mild desulfurization reagent in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, for example in the preparation of derivatives of tetrathiafulvalene
Tetrathiafulvalene
Tetrathiafulvalene is a organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, 2, by replacement of four CH groups with sulfur atoms...
.

