
Chiral Derivitizing Agent
Encyclopedia
A chiral derivatizing agent (CDA) also known as a chiral resolving reagent, is a chiral auxiliary
which can convert a mixture of enantiomer
s into diastereomer
s in order to analyse the quantities of each enantiomer present within the mix. In NMR spectroscopy
these compounds are called chiral shift reagents.
 Since NMR spectroscopy has been available to chemists, there have been numerous studies on the applications of this technique. One of these noted the difference in the chemical shift
Since NMR spectroscopy has been available to chemists, there have been numerous studies on the applications of this technique. One of these noted the difference in the chemical shift
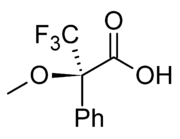
(i.e. the distance between the peaks) of two diastereomers. Conversely, two compounds that are enantiomers have the same NMR spectral properties. It was reasoned that if a mix of enantiomers could be converted into a mix of diastereomers by bonding them to another chemical that was itself chiral, it would be possible to distinguish this new mixture using NMR, and therefore learn about the original enantiomeric mixture. The first popular example of this technique was published in 1969 by Harry S. Mosher. The chiral agent is a single enantiomer of MTPA (α-methoxy-α-(trifluoromethyl)phenylacetic acid), also known as Mosher's acid
. The corresponding acid chloride is also known as Mosher's acid chloride, and the resultant diastereomeric esters are known as Mosher's esters. Another system is Pirkle's Alcohol
developed in 1977.
Since then, other methods have been developed. At first, they were based on MTPA. Later, the principles were extended to phosphorus and boron systems. Research is still continuing on in this area and the limitations of CDA's as a valid test for enantiopurity are becoming fewer as more systems are designed.
s and alcohol
s. The reason racemization does not occur is because there is no α-hydrogen near the carboxyl group (therefore it cannot form an enol
). This allows it to react with alcohol or amine to form an MTPA ester or amide respectively.
Chiral auxiliary
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers...
which can convert a mixture of enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s into diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s in order to analyse the quantities of each enantiomer present within the mix. In NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
these compounds are called chiral shift reagents.
History

Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
(i.e. the distance between the peaks) of two diastereomers. Conversely, two compounds that are enantiomers have the same NMR spectral properties. It was reasoned that if a mix of enantiomers could be converted into a mix of diastereomers by bonding them to another chemical that was itself chiral, it would be possible to distinguish this new mixture using NMR, and therefore learn about the original enantiomeric mixture. The first popular example of this technique was published in 1969 by Harry S. Mosher. The chiral agent is a single enantiomer of MTPA (α-methoxy-α-(trifluoromethyl)phenylacetic acid), also known as Mosher's acid
Mosher's acid
Mosher's acid, or α-methoxy-α-trifluoromethylphenylacetic acid is a carboxylic acid which was first used by Harry Stone Mosher as a chiral derivatizing agent. It is a chiral molecule, consisting of R and S enantiomers.-Applications:...
. The corresponding acid chloride is also known as Mosher's acid chloride, and the resultant diastereomeric esters are known as Mosher's esters. Another system is Pirkle's Alcohol
Pirkle's alcohol
Pirkle's alcohol is an off-white, crystalline solid that is stable at room temperature when protected from light and oxygen. This chiral molecule is typically used, in nonracemic form, as a chiral shift reagent in nuclear magnetic resonance spectroscopy, in order to simultaneously determine...
developed in 1977.
Since then, other methods have been developed. At first, they were based on MTPA. Later, the principles were extended to phosphorus and boron systems. Research is still continuing on in this area and the limitations of CDA's as a valid test for enantiopurity are becoming fewer as more systems are designed.
Mosher's acid
Mosher's acid or acid chloride reacts easily with alcohols and amines to give esters and amides respectively. This method is able to determine the configuration of simple chiral amineAmine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s and alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s. The reason racemization does not occur is because there is no α-hydrogen near the carboxyl group (therefore it cannot form an enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
). This allows it to react with alcohol or amine to form an MTPA ester or amide respectively.

