
Chemoluminescence
Encyclopedia

Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
with limited emission of heat (luminescence
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
), as the result of a chemical reaction. Given reactants A and B, with an excited intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
◊,
- [A] + [B] → [◊] → [Products] + lightLightLight or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
For example, if [A] is luminol
Luminol
Luminol is a versatile chemical that exhibits chemiluminescence, with a striking blue glow, when mixed with an appropriate oxidizing agent...
and [B] is hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
in the presence of a suitable catalyst we have:
- luminol + H2O2 → 3-APA[◊] → 3-APA + light
where:
- where 3-APA is 3-aminophthalate
- 3-APA[◊] is the excited state fluorescing as it decays to a lower energy level.
The decay of this excited state[◊] to a lower energy level causes light emission. In theory, one photon of light should be given off for each molecule of reactant. This is equivalent to Avogadro's number of photons per mole of reactant. In actual practice, non-enzymatic reactions seldom exceed 1% QC, quantum efficiency
Quantum efficiency
Quantum efficiency is a quantity defined for a photosensitive device such as photographic film or a charge-coupled device as the percentage of photons hitting the photoreactive surface that will produce an electron–hole pair. It is an accurate measurement of the device's electrical sensitivity to...
.
In a chemical reaction, reactants collide to form a transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
, the enthalpic maximum in a reaction coordinate diagram, which proceeds to the product. Normally, reactants form products of lesser chemical energy. The difference in energy between reactants and products, represented as
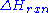 , is turned into heat, physically realized as excitations in the vibrational state of the normal modes of the product. Since vibrational energy is generally much greater than the thermal agitation, it is rapidly dispersed into the solvent through solvent molecules' rotation and translation. This is how exothermic
, is turned into heat, physically realized as excitations in the vibrational state of the normal modes of the product. Since vibrational energy is generally much greater than the thermal agitation, it is rapidly dispersed into the solvent through solvent molecules' rotation and translation. This is how exothermicExothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
reactions make their solutions hotter. In a chemiluminescent reaction, the direct product of a reaction is delivered in an excited electronic state
Electronic state
Electronic state is a quantum state of a system consisting of electrons . The state with lowest energy is called ground state, states with higher energy are excited states.See Energy level....
, which then decays into an electronic ground state through either fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
or phosphorescence
Phosphorescence
Phosphorescence is a specific type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum...
, depending on the spin state
Spin State
Spin State may refer to:* Spin quantum number, a quantum number* Spin states , the potentials for high-spin and low-spin configurations of d electrons in transition metal complexes.*Spin State, a 2003 novel by Chris Moriarty...
of the electronic excited state formed. This is possible because chemical bond formation can occur on a timescale faster than electronic transitions, and therefore can result in discrete products in excited electronic states.
Chemiluminescence differs from fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
in that the electronic excited state is derived from the product of a chemical reaction rather than the more typical way of creating electronic excited states, namely absorption
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
. It is the antithesis of a photochemical reaction, in which light is used to drive an endothermic chemical reaction. Here, light is generated from a chemically exothermic reaction.
A standard example of chemiluminescence in the laboratory setting is the luminol
Luminol
Luminol is a versatile chemical that exhibits chemiluminescence, with a striking blue glow, when mixed with an appropriate oxidizing agent...
test. Here, blood
Blood
Blood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
is indicated by luminescence
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
upon contact with iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
in hemoglobin. When chemiluminescence takes place in living organisms, the phenomenon is called bioluminescence
Bioluminescence
Bioluminescence is the production and emission of light by a living organism. Its name is a hybrid word, originating from the Greek bios for "living" and the Latin lumen "light". Bioluminescence is a naturally occurring form of chemiluminescence where energy is released by a chemical reaction in...
. A light stick emits light by chemiluminescence.
Liquid-phase reactions
- LuminolLuminolLuminol is a versatile chemical that exhibits chemiluminescence, with a striking blue glow, when mixed with an appropriate oxidizing agent...
in an alkaline solution with hydrogen peroxideHydrogen peroxideHydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
in the presence of iron or copper, or an auxiliary oxidant, produces chemiluminescence. The luminol reaction is
- luminol + H2O2 → 3-APA[◊] → 3-APA + light
Gas-phase reactions

- One of the oldest known chemoluminescent reactions is that of elemental white phosphorusPhosphorusPhosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
oxidizing in moist air, producing a green glow. This is a gas-phase reaction of phosphorus vapor, above the solid, with oxygen producing the excited states (PO)2 and HPO. - Another gas phase reaction is the basis of nitric oxideNitric oxideNitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
detection in commercial analytic instruments applied to environmental air quality testing. OzoneOzoneOzone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
is combined with nitric oxide to form nitrogen dioxide in an activated state.
- NO+O3 → NO2[◊]+ O2
- The activated NO2[◊] luminesces broadband visible to infrared light as it reverts to a lower energy state. A photomultiplierPhotomultiplierPhotomultiplier tubes , members of the class of vacuum tubes, and more specifically phototubes, are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum...
and associated electronics counts the photons which are proportional to the amount of NO present. To determine the amount of nitrogen dioxideNitrogen dioxideNitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
, NO2, in a sample (containing no NO) it must first be converted to nitric oxide, NO, by passing the sample through a converter before the above ozone activation reaction is applied. The ozone reaction produces a photon count proportional to NO which is proportional to NO2 before it was converted to NO. In the case of a mixed sample containing both NO and NO2, the above reaction yields the amount of NO and NO2 combined in the air sample, assuming that the sample is passed through the converter. If the mixed sample is not passed through the converter, the ozone reaction produces activated NO2[◊] only in proportion to the NO in the sample. The NO2 in the sample is not activated by the ozone reaction. Though unactivated NO2 is present with the activated NO2[◊], photons are only emitted by the activated species which is proportional to original NO. Final step, subtract NO from (NO + NO2) to yield NO2
Enhanced chemiluminescence
Enhanced chemiluminescence is a common technique for a variety of detection assays in biology. A horseradish peroxidaseHorseradish peroxidase
The enzyme horseradish peroxidase , found in horseradish, is used extensively in biochemistry applications primarily for its ability to amplify a weak signal and increase detectability of a target molecule.-Applications:...
enzyme (HRP) is tethered to the molecule of interest (usually through labeling an immunoglobulin that specifically recognizes the molecule). This enzyme complex, then catalyzes the conversion of the enhanced chemiluminescent substrate into a sensitized reagent in the vicinity of the molecule of interest, which on further oxidation by hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, produces a triplet (excited) carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
which emits light when it decays to the singlet carbonyl. Enhanced chemiluminescence allows detection of minute quantities of a biomolecule. Proteins can be detected down to femtomole quantities, well below the detection limit for most assay systems.
Applications
- Gas analysis: for determining small amounts of impurities or poisons in air. Other compounds can also be determined by this method (ozoneOzoneOzone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
, N-oxides, S-compounds). A typical example is NO determination with detection limits down to 1 ppb - Analysis of inorganic species in liquid phase
- Analysis of organic species: useful with enzymeEnzymeEnzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s, where the substrate isn't directly involved in chemiluminescence reaction, but the product is - Detection and assay of biomolecules in systems such as ELISAELISAEnzyme-linked immunosorbent assay , is a popular format of a "wet-lab" type analytic biochemistry assay that uses one sub-type of heterogeneous, solid-phase enzyme immunoassay to detect the presence of a substance in a liquid sample."Wet lab" analytic biochemistry assays involves detection of an...
and Western blotWestern blotThe western blot is a widely used analytical technique used to detect specific proteins in the given sample of tissue homogenate or extract. It uses gel electrophoresis to separate native proteins by 3-D structure or denatured proteins by the length of the polypeptide...
s - DNA sequencingDNA sequencingDNA sequencing includes several methods and technologies that are used for determining the order of the nucleotide bases—adenine, guanine, cytosine, and thymine—in a molecule of DNA....
using pyrosequencingPyrosequencingPyrosequencing is a method of DNA sequencing based on the "sequencing by synthesis" principle. It differs from Sanger sequencing, in that it relies on the detection of pyrophosphate release on nucleotide incorporation, rather than chain termination with dideoxynucleotides... - Lighting objects. Chemiluminescence kitesKite typesKites are tethered flying objects which fly by using aerodynamic lift, requiring wind, , for generation of airflow over the lifting surfaces.-Kite types:...
, emergency lighting, glow sticks (party decorations). - Combustion analysis: certain radical species (such as CH* and OH*) give off radiation at specific wavelengths. The heat release rate is calculated by measuring the amount of light radiated from a flame at those wavelengths.
- Children's toys

