
Catecholborane
Encyclopedia
Catecholborane, or HBcat, is a derivative of catechol and a boron
hydride
, with the formula C6H4O2BH. It is commonly used in organic syntheses
.
(BH3) in a cooled solution of THF
. However, this method results in a loss of 2 mole equivalents of the hydride.
Heinrich Noth and Detlef Mannig devised a method that allows a more efficient recovery of the final product, catecholborane, and subsequently a greater yield. Their method involves reacting an alkali-metal boron hydride (LiBH4, NaBH4, of KBH4) with tris(catecholato)bisborane in an ethereal solvent such as diethyl ether
.
In 2001 Herbert Brown released an additional procedure for catecholborane synthesis. His method involves reacting tri-O-phenylene bis-borate with diborane
in a solution of either triglyme or tetraglyme. Brown claimed his method produces 85% yield of 97% pure product, catecholborane.
, usually a terminal alkyne, through hydroboration a trans vinylborane is formed. This is the precursor to the Suzuki reaction
.

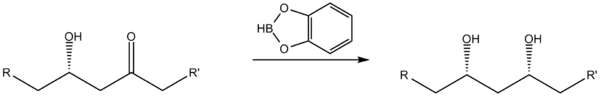
when converting β-hydroxy ketones to syn 1,3-diols.
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
, with the formula C6H4O2BH. It is commonly used in organic syntheses
Organic Syntheses
Organic Syntheses is a scientific journal that since 1921 has provided the chemistry community with annual collections of detailed and checked procedures for the organic synthesis of organic compounds. The journal is peer reviewed...
.
Synthesis
Traditionally catecholborane commonly is produced by reacting catechol with boraneBorane
In chemistry, a borane is a chemical compound of boron and hydrogen. The boranes comprise a large group of compounds with the generic formulae of BxHy. These compounds do not occur in nature. Many of the boranes readily oxidise on contact with air, some violently. The parent member BH3 is called...
(BH3) in a cooled solution of THF
ThF
Follicular B helper T cells , are antigen-experienced CD4+ T cells found in the B cell follicles of secondary lymphoid organs such as lymph nodes, spleens and Peyer's patches, and are identified by their constitutive expression of the B cell follicle homing receptor CXCR5...
. However, this method results in a loss of 2 mole equivalents of the hydride.
Heinrich Noth and Detlef Mannig devised a method that allows a more efficient recovery of the final product, catecholborane, and subsequently a greater yield. Their method involves reacting an alkali-metal boron hydride (LiBH4, NaBH4, of KBH4) with tris(catecholato)bisborane in an ethereal solvent such as diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
.
In 2001 Herbert Brown released an additional procedure for catecholborane synthesis. His method involves reacting tri-O-phenylene bis-borate with diborane
Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
in a solution of either triglyme or tetraglyme. Brown claimed his method produces 85% yield of 97% pure product, catecholborane.
Preparation of an organoborane
When catecholborane is reacted with an alkyneAlkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
, usually a terminal alkyne, through hydroboration a trans vinylborane is formed. This is the precursor to the Suzuki reaction
Suzuki reaction
The Suzuki reaction is the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium complex. It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls, and has been extended to incorporate alkyl bromides...
.

Reduction of β-hydroxy ketones
Catecholborane may be used as a stereoselective reducing agentReducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
when converting β-hydroxy ketones to syn 1,3-diols.


