
Bicarbonate buffering system
Encyclopedia
The bicarbonate buffering system is an important buffer
system in the acid-base homeostasis
of living things, including humans. As a buffer, it tends to maintain a relatively constant plasma
pH
and counteract any force that would alter it.
In this system, carbon dioxide
(CO2) combines with water
to form carbonic acid
(H2CO3), which in turn rapidly dissociates to form hydrogen ion
and bicarbonate
(HCO3- ) as shown in the reactions below. The carbon dioxide - carbonic acid equilibrium is catalyzed
by the enzyme
carbonic anhydrase
; the carbonic acid - bicarbonate equilibrium is simple proton dissociation/association and needs no catalyst.
Any disturbance of the system will be compensated by a shift in the chemical equilibrium
according to Le Chatelier's principle
. For example, if one attempted to acidify the blood by dumping in an excess of hydrogen ions (acidemia), some of those hydrogen ions will associate with bicarbonate, forming carbonic acid, resulting in a smaller net increase of acidity than otherwise. This buffering system becomes an even more powerful regulator of acidity when it is coupled with the body's capacity for respiratory compensation
, in which breathing
is altered to modify the amount of CO2 in circulation. In the above example, increased ventilation would increase the loss of CO2 to the atmosphere, driving the equilibria above to the left. The process could continue until the excess acid is all exhaled
.
This process is extremely important in the physiology
of blood-having animals. It manages the many acid and base imbalances
that can be produced by both normal and abnormal physiology
. It also affects the handling of carbon dioxide, the constantly produced waste product of cellular respiration
.
to constituents of the bicarbonate buffering system:
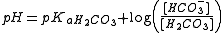
, where:
This is useful in arterial blood gas
, but these usually state pCO2, that is, the partial pressure
of carbon dioxide
, rather than H2CO3. However, these are related by the equation:

, where:
Taken together, the following equation can be used to relate the pH of blood to the concentration of bicarbonate and the partial pressure of carbon dioxide:
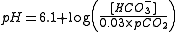
, where:
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
system in the acid-base homeostasis
Acid-base homeostasis
Acid–base homeostasis is the part of human homeostasis concerning the proper balance between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so strong mechanisms exist to maintain it...
of living things, including humans. As a buffer, it tends to maintain a relatively constant plasma
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
and counteract any force that would alter it.
In this system, carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2) combines with water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
to form carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
(H2CO3), which in turn rapidly dissociates to form hydrogen ion
Hydrogen ion
Hydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
and bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
(HCO3- ) as shown in the reactions below. The carbon dioxide - carbonic acid equilibrium is catalyzed
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
by the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
carbonic anhydrase
Carbonic anhydrase
The carbonic anhydrases form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons , a reversible reaction that occurs rather slowly in the absence of a catalyst...
; the carbonic acid - bicarbonate equilibrium is simple proton dissociation/association and needs no catalyst.

Any disturbance of the system will be compensated by a shift in the chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
according to Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
. For example, if one attempted to acidify the blood by dumping in an excess of hydrogen ions (acidemia), some of those hydrogen ions will associate with bicarbonate, forming carbonic acid, resulting in a smaller net increase of acidity than otherwise. This buffering system becomes an even more powerful regulator of acidity when it is coupled with the body's capacity for respiratory compensation
Respiratory compensation
Respiratory compensation is a mechanism by which plasma pH can be altered by varying the respiratory rate. It is faster than renal compensation, but has less ability to restore normal values....
, in which breathing
Breathing
Breathing is the process that moves air in and out of the lungs. Aerobic organisms require oxygen to release energy via respiration, in the form of the metabolism of energy-rich molecules such as glucose. Breathing is only one process that delivers oxygen to where it is needed in the body and...
is altered to modify the amount of CO2 in circulation. In the above example, increased ventilation would increase the loss of CO2 to the atmosphere, driving the equilibria above to the left. The process could continue until the excess acid is all exhaled
Exhalation
Exhalation is the movement of air out of the bronchial tubes, through the airways, to the external environment during breathing....
.
This process is extremely important in the physiology
Physiology
Physiology is the science of the function of living systems. This includes how organisms, organ systems, organs, cells, and bio-molecules carry out the chemical or physical functions that exist in a living system. The highest honor awarded in physiology is the Nobel Prize in Physiology or...
of blood-having animals. It manages the many acid and base imbalances
Acid-base imbalance
Acid–base imbalance is an abnormality of the human body's normal balance of acids and bases that causes the plasma pH to deviate out of the normal range . In the fetus, the normal range differs based on which umbilical vessel is sampled...
that can be produced by both normal and abnormal physiology
Pathophysiology
Pathophysiology is the study of the changes of normal mechanical, physical, and biochemical functions, either caused by a disease, or resulting from an abnormal syndrome...
. It also affects the handling of carbon dioxide, the constantly produced waste product of cellular respiration
Cellular respiration
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate , and then release waste products. The reactions involved in respiration are catabolic reactions that involve...
.
Henderson-Hasselbach equation
A modified version of the Henderson-Hasselbach equation can be used to relate the pH of bloodBlood
Blood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
to constituents of the bicarbonate buffering system:
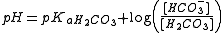
, where:
- pKa H2CO3 is the acid dissociation constantAcid dissociation constantAn acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of carbonic acidCarbonic acidCarbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
. It is equal to 6.1. - [HCO3-] is the concentration of bicarbonateBicarbonateIn inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
in the blood - [H2CO3] is the concentration of carbonic acid in the blood
This is useful in arterial blood gas
Arterial blood gas
An arterial blood gas is a blood test that is performed using blood from an artery. It involves puncturing an artery with a thin needle and syringe and drawing a small volume of blood. The most common puncture site is the radial artery at the wrist, but sometimes the femoral artery in the groin or...
, but these usually state pCO2, that is, the partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, rather than H2CO3. However, these are related by the equation:

, where:
- [H2CO3] is the concentration of carbonic acid in the blood
- kH CO2 is a constant including the solubilitySolubilitySolubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
of carbon dioxide in blood. kH CO2 is approximately 0.03 mmol/mmHg - pCO2 is the partial pressurePartial pressureIn a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
in the blood
Taken together, the following equation can be used to relate the pH of blood to the concentration of bicarbonate and the partial pressure of carbon dioxide:
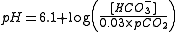
, where:
- pH is the acidity in the blood
- [HCO3-] is the concentration of bicarbonate in the blood
- pCO2 is the partial pressure of carbon dioxide in the blood

