
BglII
Encyclopedia

Endonuclease
Endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain, in contrast to exonucleases, which cleave phosphodiester bonds at the end of a polynucleotide chain. Typically, a restriction site will be a palindromic sequence four to six nucleotides long. Most...
enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
isolated from certain strains of Bacillus globigii. The principal function of restriction enzymes is the protection of the host genome against foreign DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
but they may also have some involvement in recombination
Genetic recombination
Genetic recombination is a process by which a molecule of nucleic acid is broken and then joined to a different one. Recombination can occur between similar molecules of DNA, as in homologous recombination, or dissimilar molecules, as in non-homologous end joining. Recombination is a common method...
and transposition
Transposition
Transposition may refer to:Mathematics* Transposition , a permutation which exchanges two elements and keeps all others fixed* Transposition, producing the transpose of a matrix AT, which is computed by swapping columns for rows in the matrix AGames* Transposition , different moves or a different...
. Like most type II restriction enzymes BglII consists of two identical subunits that form a homodimer
Protein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
around the DNA double helix. Each monomer is 223 amino acids and symmetrically bind both sides of the unique palindromic nucleotide sequence AGATCT, cleaving the scissile phosphodiester bond between the first Adenine and Guanine nucleotides on both strands of the DNA molecule creating sticky ends with 5' end overhangs. Being a type II restriction enzyme BglII does not require ATP (adenosine triphosphate
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
) for its enzymatic function but only requires association with a divalent
Divalent
In chemistry, a divalent ion or molecule has a valence of two and thus can form two bonds with other ions or molecules. An older term for divalent is bivalent....
metal cation, most likely Mg2+. Unlike other restriction enzymes of its class, BglII has been show to possess some unique structural characteristics such as a β-sandwich subdomain and appears to undergo a unique conformational change
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
upon dimerization, but it’s overall structure and mechanism of catalysis remain consistent with other type II restriction enzymes. Restriction Endonuclease enzymes play a very important role in modern molecular cloning
Molecular cloning
Molecular cloning refers to a set of experimental methods in molecular biology that are used to assemble recombinant DNA molecules and to direct their replication within host organisms...
techniques. Because of their unique recognition/cut sites restriction enzymes can be used to precisely and controlably cut DNA in specific locations. Once cut, scientist can then insert a desired DNA fragment that contains complementary "sticky ends" into the linearized DNA and ligate them together to create an engineered cloning vector
Cloning vector
A cloning vector is a small piece of DNA into which a foreign DNA fragment can be inserted. The insertion of the fragment into the cloning vector is carried out by treating the vehicle and the foreign DNA with a restriction enzyme that creates the same overhang, then ligating the fragments...
.
| Identifiers | |
| Name | Bgl II Restriction Endonuclease |
| Entrez | 6173168 |
| PDB | 1DFM |
| ACCESSION # | Q45488 |
| EC Number | 3.1.21.4 |
Mechanism
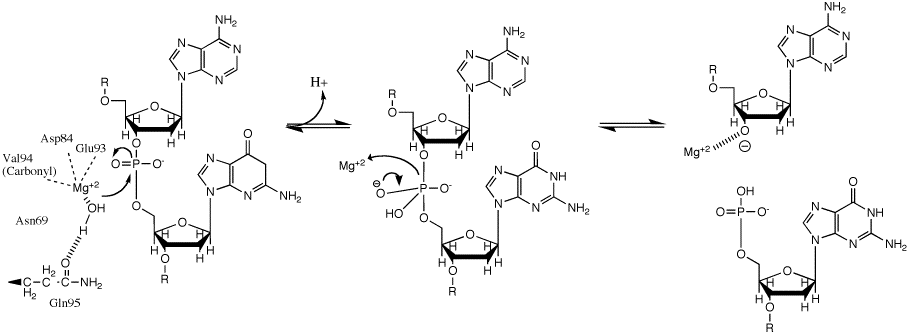 |
This phosphoryl transfer occurs by a nucleophilic attack of a hydride ion on the scissile phosphate, resulting in a trigonal bipyramidal phosphorus intermediate. The phosphorus then gets substituted and the 3'-0- is kicked off as a leaving group. |
BglII catalyses phosphodiester bond
Phosphodiester bond
A phosphodiester bond is a group of strong covalent bonds between a phosphate group and two 5-carbon ring carbohydrates over two ester bonds. Phosphodiester bonds are central to all known life, as they make up the backbone of each helical strand of DNA...
cleavage at the DNA backbone through a phosphoryl transfer to water. Studies on the mechanism of restriction enzymes have revealed several general features that seem to be true in almost all cases, although the actual mechanism for each enzyme is most likely some variation of this general mechanism. This mechanism requires a base to generate the hydroxide ion from water, which will act as the nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
and attack the phosphorus in the phosphodiester bond. Also required is a Lewis acid to stabilize the extra negative charge of the pentacoordinated transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
phosphorus as well as a general acid or metal ion that stabilizes the leaving group (3’-O-).
Structure

Comparative structural studies of the free enzyme vs. the BglII-DNA complex showed that the enzyme opens by a dramatic scissor-like motion, accompanied by a complete rearrangement of the α-helicies at the dimer interface. These structural studies also revealed that within each monomer a set of residues lowers or raises to alternatively sequester or expose the active site residues. These dramatic differences in structure in the free vs. bound enzyme have yet to be observed in any other restriction endonuclease and may possibly represent a novel mechanism for capturing DNA that may extend to other proteins that encircle DNA.
Active site
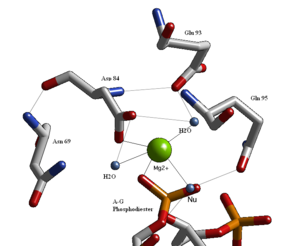
Structural studies of endonucleases have revealed a similar architecture for the [active site] with the residues following the weak consensus sequence (Glu/Asp)-X(9-20)-(Glu/Asp/Ser)-X-(Lys/Glu). BglII active site is similar to other endonucleases but follows the sequence Asp 84-X9-Glu 93-X-Gln 95. In its active site there sits a divalent metal ion, most likely Mg2+, that allows interactions with Asp 84, Val 94, a phosphorus oxygen, and three water molecules. One of these water molecules, which we have labeled Nu, is well equipped to be the source of the hydroxide nucleophile because of its proximity to the scissile phosphate as well as a pKa lowered by its contact with the metal and its orientation fixed by a hydrogen bond with the side chain Oxygen of Gln 95.
See also
- BamHIBamHIBamHI is a restriction enzyme, derived from Bacillus amyloliquefaciens. It has the recognition site , and leaves a sticky end. One of the earlier enzymes to be used, it is popular for historical reasons, but also because digestion leaves a GATC overhang compatible with many other enzymes...
, a nuclease enzyme from Bacillus amyloliquefaciens.. - FokIFokIThe enzyme FokI, naturally found in Flavobacterium okeanokoites, is a bacterial type IIS restriction endonuclease consisting of an N-terminal DNA-binding domain and a non-specific DNA cleavage domain at the C-terminal...
, a nuclease enzyme from Flavobacterium okeanokoites - EcoRIEcoRIEcoRI is an endonuclease enzyme isolated from strains of E. coli, and is part of the restriction modification system.In molecular biology it is used as a restriction enzyme. It creates sticky ends with 5' end overhangs...
, a nuclease enzyme from E. coli.

