Beta-hydride elimination
Encyclopedia
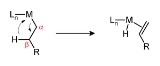
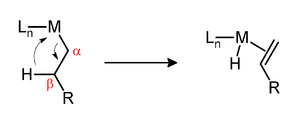
Beta-hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride
and an alkene
. The alkyl must have hydrogens on the beta carbon. For instance butyl
groups can undergo this reaction but methyl groups cannot. The metal complex must have an empty (or vacant) site cis to the alkyl group for this reaction to occur.
 The beta-hydride elimination can either be a vital step in a reaction or an unproductive side reaction. The Shell higher olefin process
The beta-hydride elimination can either be a vital step in a reaction or an unproductive side reaction. The Shell higher olefin process
relies on beta-hydride elimination to produce alpha-olefins which are used to produce detergents. Illustrative of a sometimes undesirable beta-hydride elimination, beta-hydride elimination in Ziegler-Natta polymerization results in polymers of decreased molecular weight. In the case of nickel
- and palladium
-catalyzed couplings
of aryl halides with alkyl Grignard reagents, the beta-hydride elimination can lower the yield. The production of branched polymers from ethylene relies on chain walking
, a key step of which is beta-hydride elimination.
In some cases, beta-hydride elimination is the first in a series of steps. For instance in the synthesis of RuHCl(CO)(PPh3)3 from ruthenium trichloride, triphenylphosphine
and 2-methoxyethanol
, an intermediate alkoxide
complex undergoes a beta-hydride elimination to form the hydride
ligand and the pi-bonded aldehyde
which then is later converted into the carbonyl
(carbon monoxide
) ligand.
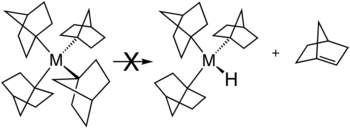 Bulky alkyl ligands, such as t-butyl or trimethylsilyl, may prohibit the hydrogen atom from approaching a coplanar configuration with respect to the metal, and the α and β atoms. If the metal center does not have empty coordination sites, for example, by the complex already having 18 electron configuration, β-hydride elimination is not possible as well.
Bulky alkyl ligands, such as t-butyl or trimethylsilyl, may prohibit the hydrogen atom from approaching a coplanar configuration with respect to the metal, and the α and β atoms. If the metal center does not have empty coordination sites, for example, by the complex already having 18 electron configuration, β-hydride elimination is not possible as well.
In some cases, the coligands can impose geometries that inhibit beta-hydride elimination. For the above example, the unwanted beta-hydride elimination is prevented by using a diphosphine where the two phosphorus
atoms are fixed apart in space. One way of doing this is to use a ferrocene
unit. The nickel and palladium complexes of 1,1'-diphosphinoferrocenes are arranged such that the metal has two phosphorus atoms in the trans sites. As these metals form square planar
complexes, no vacant site cis to the alkyl group can be formed. Hence the beta-hydride elimination is prevented. (see trans-spanning ligand
)
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
and an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. The alkyl must have hydrogens on the beta carbon. For instance butyl
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula -C4H9, derived from either of the two isomers of butane....
groups can undergo this reaction but methyl groups cannot. The metal complex must have an empty (or vacant) site cis to the alkyl group for this reaction to occur.

Shell higher olefin process
The Shell higher olefin process is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Royal Dutch Shell. The olefin products are converted to fatty aldehydes and then to fatty alcohols, which are precursors...
relies on beta-hydride elimination to produce alpha-olefins which are used to produce detergents. Illustrative of a sometimes undesirable beta-hydride elimination, beta-hydride elimination in Ziegler-Natta polymerization results in polymers of decreased molecular weight. In the case of nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
- and palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
-catalyzed couplings
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
of aryl halides with alkyl Grignard reagents, the beta-hydride elimination can lower the yield. The production of branched polymers from ethylene relies on chain walking
Chain walking
Chain walking or chain running is a mechanism that operates during some alkene polymerization reactions. This reaction gives rise to branched and hyperbranched hydrocarbon polymers. This process is also characterized by accurate control of polymer architecture and topology. The positions of...
, a key step of which is beta-hydride elimination.
In some cases, beta-hydride elimination is the first in a series of steps. For instance in the synthesis of RuHCl(CO)(PPh3)3 from ruthenium trichloride, triphenylphosphine
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
and 2-methoxyethanol
2-Methoxyethanol
2-Methoxyethanol, or methyl cellosolve, is an organic compound that is used mainly as a solvent. It is a clear, colorless liquid with an ether-like odor. It is in a class of solvents known as glycol ethers which are notable for their ability to dissolve a variety of different types of chemical...
, an intermediate alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
complex undergoes a beta-hydride elimination to form the hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
ligand and the pi-bonded aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
which then is later converted into the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
(carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
) ligand.
Avoiding β-hydride elimination
Several strategies exist for avoiding β-hydride elimination. The most common strategy is to employ alkyl ligands that lack a beta-hydrogen centres in the first place. Common substituents include methyl and neopentyl. Beta-hydride elimination is also inhibited when the reaction would produce a strained alkene. This situation is illustrated by the stability of metal complexes containing norbornyl ligands.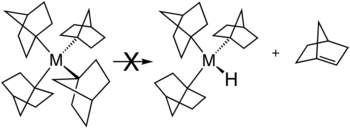
In some cases, the coligands can impose geometries that inhibit beta-hydride elimination. For the above example, the unwanted beta-hydride elimination is prevented by using a diphosphine where the two phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
atoms are fixed apart in space. One way of doing this is to use a ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
unit. The nickel and palladium complexes of 1,1'-diphosphinoferrocenes are arranged such that the metal has two phosphorus atoms in the trans sites. As these metals form square planar
Square planar
The square planar molecular geometry in chemistry describes the stereochemistry that is adopted by certain chemical compounds...
complexes, no vacant site cis to the alkyl group can be formed. Hence the beta-hydride elimination is prevented. (see trans-spanning ligand
Trans-spanning ligand
Trans-spanning ligands are bidentate ligands that can span opposite sites of a complex with square-planar geometry. A wide variety of ligands that chelate in the cis fashion already exist, but very few can link opposite vertices on a coordination polyhedron...
)

