Atmospheric window
Encyclopedia
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
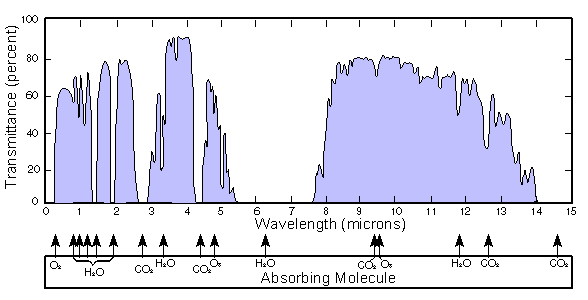
, taken as a whole at each place and occasion of interest, that lets some infrared radiation from the cloud tops and land-sea surface pass directly to space without intermediate absorption and re-emission, and thus without heating the atmosphere. It cannot be defined simply as a part or set of parts of the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
, because the spectral composition of window radiation varies greatly with varying local environmental conditions, such as water vapour content and land-sea surface temperature, and because few or no parts of the spectrum are simply not absorbed at all, and because some of the diffuse radiation is passing nearly vertically upwards and some is passing nearly horizontally. A large gap in the absorption spectrum of water vapor, the main greenhouse gas, is most important in the dynamics of the window. Other gases, especially carbon dioxide and ozone, partly block transmission.
One should take care to distinguish between the atmospheric window and the spectral window. An atmospheric window is a dynamic property of the atmosphere, while the spectral window is a static characteristic of the electromagnetic radiative absorption spectra of many greenhouse gases, including water vapour. The atmospheric window tells what actually happens in the atmosphere, while the spectral window tells of one of the several abstract factors that potentially contribute to the actual concrete happenings in the atmosphere.
It is also important to distinguish between the terms window radiation and radiation of window wavelength (window wavelength radiation). Window radiation is radiation that actually passes through the atmospheric window. Non-window radiation is radiation that actually does not pass through the atmospheric window. Window wavelength radiation is radiation that, judging only from its wavelength, potentially might or might not, but is likely to pass through the atmospheric window. Non-window wavelength radiation is radiation that, judging only from its wavelength, is unlikely to pass through the atmospheric window. The difference between window radiation and window wavelength radiation is that window radiation is an actual component of the radiation, determined by the full dynamics of the atmosphere, taking in all determining factors, while window wavelength radiation is merely theoretically potential, defined only by one factor, the wavelength.
The importance of the infrared atmospheric window in the atmospheric energy balance was discovered by George Simpson
George Simpson (meteorologist)
Sir George Clarke Simpson KCB CBE FRS was a British meteorologist, born in Derby, England.-Biography:George Clarke Simpson was born 2 September 1878 in Derby England, the son of Arthur Simpson, a proprietor of a department store in East Street, and Alice Lambton Clarke...
in 1928, based on G. Hettner's 1918 laboratory studies of the gap in the absorption spectrum of water vapor. In those days, computers were not available, and Simpson notes that he used approximations; he writes: "There is no hope of getting an exact solution; but by making suitable simplifying assumptions . . . ." Nowadays, accurate line-by-line computations are possible, and careful studies of the infrared atmospheric window have been published.
Kinetics of the infrared atmospheric window
The infrared atmospheric window is a path from the land-sea surface of the earth to space. It separates two radiative components, window and non-window radiation, that are not of the kind that have kinetics suitable for description by the Beer-Lambert lawBeer-Lambert law
In optics, the Beer–Lambert law, also known as Beer's law or the Lambert–Beer law or the Beer–Lambert–Bouguer law relates the absorption of light to the properties of the material through which the light is travelling.-Equations:The law states that there is a logarithmic dependence between the...
. The window radiation and the non-window radiation from the land-sea surface are not defined in the terms that are necessary for the application of the Beer-Lambert Law. It would therefore be a logical and conceptual error to try to apply the Beer-Lambert Law either to window or non-window radiation considered separately.
The reason for this is that the window and non-window radiation have already been conditioned by the Beer-Lambert Law and the law cannot validly be re-applied to its own products. Logically, the Beer-Lambert Law applies to radiation of which the origin is known but the destination is unknown. Such is not the case for window and non-window radiation. Logically, it is part of the definition of window radiation that its destination is known, namely that it is destined to go to space, and likewise, by definition the destination of non-window radiation is known to be entire absorption by the atmosphere. Thus it makes sense to state the precise spectral distribution and spatial, especially altitudinal, distribution of locations of absorption of non-window radiation in the atmosphere. But none of those locations can be beyond the atmosphere; by definition, non-window radiation has zero probability of escaping absorption by the atmosphere; all of the locations of absorption are within the atmosphere. Radiation that can be described by the Beer-Lambert Law can partly escape absorption by the medium of interest; the law tells just how much that part is. This is a deep conceptual point that distinguishes the kinetic description of window and non-window radiation from the kinetic description of the kind of radiation that is covered by the Beer-Lambert Law.
Non-window radiation is by definition absorbed by the atmosphere, and its energy is thereby transduced into kinetic energy of atmospheric molecules. That kinetic energy is then transferred according to the usual dynamics of atmospheric energy transfer.
These kinetic principles for window and non-window radiation arise in the light of the definition of the atmospheric window as a dynamic property of the whole atmosphere, logically distinct from the electromagnetic spectral window.
Mechanisms in the infrared atmospheric window
The infrared absorptions of the principal natural greenhouse gasGreenhouse gas
A greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
es are mostly in two ranges. At wavelengths longer than 14 µm (micrometres), gases such as CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and CH4
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
(along with less abundant hydrocarbons) absorb due to the presence of relatively long C-H and carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
, as well as water (H2O) vapor absorbing in rotation modes. The bonds of H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
and NH3
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
absorb at wavelengths shorter than 8 µm. Except for the bonds in O3
Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
, no bonds between carbon, hydrogen, oxygen and nitrogen atoms absorb in the interval between about 8 and 14 µm, though there is weaker continuum absorption in that interval.
Over the Atlas Mountains
Atlas Mountains
The Atlas Mountains is a mountain range across a northern stretch of Africa extending about through Morocco, Algeria, and Tunisia. The highest peak is Toubkal, with an elevation of in southwestern Morocco. The Atlas ranges separate the Mediterranean and Atlantic coastlines from the Sahara Desert...
, interferometrically recorded spectra of outgoing longwave radiation show emission that has arisen from the land surface at a temperature of about 320 K and passed through the atmospheric window, and non-window emission that has arisen mainly from the troposphere at temperatures about 260 K.
Over the Ivory Coast, interferometrically recorded spectra of outgoing longwave radiation show emission that has arisen from the cloud tops at a temperature of about 265 K and passed through the atmospheric window, and non-window emission that has arisen mainly from the troposphere at temperatures about 240 K.
This means that, at the scarcely absorbed continuum of wavelengths (8 to 14 µm), the radiation emitted, by the earth's surface into a dry atmosphere, and by the cloud tops, mostly passes unabsorbed through the atmosphere, and is emitted directly to space; there is also partial window transmission in far infrared spectral lines between about 16 and 28 µm. Clouds are excellent emitters of infrared radiation. Window radiation from cloud tops arises at altitudes where the air temperature is low, but as seen from those altitudes, the water vapor content of the air above is much lower than that of the air at the land-sea surface. Moreover, the water vapour continuum absorptivity, molecule for molecule, decreases with pressure decrease. Thus water vapour above the clouds, besides being less concentrated, is also less absorptive than water vapour at lower altitudes. Consequently, the effective window as seen from the cloud-top altitudes is more open, with the result that the cloud tops are effectively strong sources of window radiation; that is to say, in effect the clouds obstruct the window only to a small degree (see another opinion about this, proposed by Ahrens (2009) on page 43).
Importance for life
Without the infrared atmospheric window, the Earth would become much too warm to support life, and possibly so warm that it would lose its water as VenusVenus
Venus is the second planet from the Sun, orbiting it every 224.7 Earth days. The planet is named after Venus, the Roman goddess of love and beauty. After the Moon, it is the brightest natural object in the night sky, reaching an apparent magnitude of −4.6, bright enough to cast shadows...
did early in solar system
Solar System
The Solar System consists of the Sun and the astronomical objects gravitationally bound in orbit around it, all of which formed from the collapse of a giant molecular cloud approximately 4.6 billion years ago. The vast majority of the system's mass is in the Sun...
history. Thus, the existence of an atmospheric window is critical to Earth remaining a habitable planet
Planetary habitability
Planetary habitability is the measure of a planet's or a natural satellite's potential to sustain life. Life may develop directly on a planet or satellite or be transferred to it from another body, a theoretical process known as panspermia...
.
Threats
In recent decades, the existence of the infrared atmospheric window has become threatened by the development of highly unreactive gases containing bonds between fluorineFluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
and either carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
or sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. The "stretching frequencies" of bonds between fluorine and other light nonmetal
Nonmetal
Nonmetal, or non-metal, is a term used in chemistry when classifying the chemical elements. On the basis of their general physical and chemical properties, every element in the periodic table can be termed either a metal or a nonmetal...
s are such that strong absorption in the atmospheric window will always be characteristic of compounds containing such bonds. This absorption is strengthened because these bonds are highly polar due to the extreme electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of the fluorine atom. Bonds to other halogens also absorb in the atmospheric window, though much less strongly.
Moreover, the unreactive nature of such compounds that makes them so valuable for many industrial purposes means that they are not removable in the natural circulation of the Earth's atmosphere. It is estimated, for instance, that perfluorocarbons (CF4, C2F6, C3F8) can stay in the atmosphere for over fifty thousand years, a figure which may be an underestimate given the absence of natural sources of these gases.
This means that such compounds have an enormous global warming potential. One kilogram of sulfur hexafluoride
Sulfur hexafluoride
Sulfur hexafluoride is an inorganic, colorless, odorless, and non-flammable greenhouse gas. has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule. Typical for a nonpolar gas, it is poorly soluble in water but soluble in...
will, for example, cause as much warming as 23 tonnes of carbon dioxide over 100 years. Perfluorocarbons are similar in this respect, and even carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
(CCl4) has a global warming potential of 1800 compared to carbon dioxide.
Efforts to find substitutes for these compounds are still going on and remain highly problematic.
See also
- Astronomical window
- Greenhouse effectGreenhouse effectThe greenhouse effect is a process by which thermal radiation from a planetary surface is absorbed by atmospheric greenhouse gases, and is re-radiated in all directions. Since part of this re-radiation is back towards the surface, energy is transferred to the surface and the lower atmosphere...
- Greenhouse gasGreenhouse gasA greenhouse gas is a gas in an atmosphere that absorbs and emits radiation within the thermal infrared range. This process is the fundamental cause of the greenhouse effect. The primary greenhouse gases in the Earth's atmosphere are water vapor, carbon dioxide, methane, nitrous oxide, and ozone...
- Optical windowOptical windowThe meaning of this term depends on the context:* In astronomy, the optical window is the optical portion of the electromagnetic spectrum that passes through the atmosphere all the way to the ground...
- Ozone depletionOzone depletionOzone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
- Radio windowRadio windowThe radio window is the range of frequencies of electromagnetic radiation that the earth's atmosphere lets through. The wavelengths in the radio window run from about one centimetre to about eleven-metre waves.-See also:*Astronomical window...

