
Anthrax toxin
Encyclopedia

Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
exotoxin
Exotoxin
An exotoxin is a toxin excreted by a microorganism, like bacteria, fungi, algae, and protozoa. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host...
secreted by virulent strains of the bacterium, Bacillus anthracis
Bacillus anthracis
Bacillus anthracis is the pathogen of the Anthrax acute disease. It is a Gram-positive, spore-forming, rod-shaped bacterium, with a width of 1-1.2µm and a length of 3-5µm. It can be grown in an ordinary nutrient medium under aerobic or anaerobic conditions.It is one of few bacteria known to...
--the causative agent of anthrax
Anthrax
Anthrax is an acute disease caused by the bacterium Bacillus anthracis. Most forms of the disease are lethal, and it affects both humans and other animals...
. The toxin was first discovered by Harry Smith
Harold Hill Smith
Harold Hill Smith was an American geneticist who first fused a human cell and a plant cell.-Life and career:...
in 1954. Anthrax toxin is composed of a cell-binding protein, known as protective antigen
Antigen
An antigen is a foreign molecule that, when introduced into the body, triggers the production of an antibody by the immune system. The immune system will then kill or neutralize the antigen that is recognized as a foreign and potentially harmful invader. These invaders can be molecules such as...
(PA), and two enzyme components, called edema factor (EF) and lethal factor
Anthrax lethal factor endopeptidase
Anthrax lethal factor endopeptidase is an enzyme that catalyzes the hydrolysis of mitogen-activated protein kinase kinases. This enzyme is a component of the lethal factor produced by the bacterium Bacillus anthracis. The preferred cleavage site can be denoted by BBBBxHxH, in which B denotes a...
(LF). These three protein components act together to impart their physiological effects. Assembled complexes containing the toxin components are endocytosed
Endocytosis
Endocytosis is a process by which cells absorb molecules by engulfing them. It is used by all cells of the body because most substances important to them are large polar molecules that cannot pass through the hydrophobic plasma or cell membrane...
. In the endosome
Endosome
In biology, an endosome is a membrane-bound compartment inside eukaryotic cells. It is a compartment of the endocytic membrane transport pathway from the plasma membrane to the lysosome. Molecules internalized from the plasma membrane can follow this pathway all the way to lysosomes for...
, the enzymatic components of the toxin translocate into the cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
of a target cell. Once in the cytosol, the enzymatic components of the toxin disrupts various immune cell functions, namely cellular signaling and cell migration. The toxin may even induce cell lysis, as is observed for macrophage
Macrophage
Macrophages are cells produced by the differentiation of monocytes in tissues. Human macrophages are about in diameter. Monocytes and macrophages are phagocytes. Macrophages function in both non-specific defense as well as help initiate specific defense mechanisms of vertebrate animals...
cells. Anthrax toxin ultimately allows the bacteria to evade the immune system
Immune system
An immune system is a system of biological structures and processes within an organism that protects against disease by identifying and killing pathogens and tumor cells. It detects a wide variety of agents, from viruses to parasitic worms, and needs to distinguish them from the organism's own...
, proliferate, and ultimately kill the host animal. Research on anthrax toxin also provides insight on macromolecular assembly, protein translocation, pore formation, endocytosis
Endocytosis
Endocytosis is a process by which cells absorb molecules by engulfing them. It is used by all cells of the body because most substances important to them are large polar molecules that cannot pass through the hydrophobic plasma or cell membrane...
, and other biochemical
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
processes.
Bacillus anthracis virulence factors
Anthrax is a disease caused by Bacillus anthracis, a spore-forming, Gram positive, rod-shaped bacterium (Fig. 1). The lethality of the disease is caused by the bacterium's two principal virulence factors: (i) the polyglutamic acid capsule, which is anti-phagocytic, and (ii) the tripartite protein toxin, called anthrax toxin. Anthrax toxin is a mixture of three protein components: (i) protective antigenAntigen
An antigen is a foreign molecule that, when introduced into the body, triggers the production of an antibody by the immune system. The immune system will then kill or neutralize the antigen that is recognized as a foreign and potentially harmful invader. These invaders can be molecules such as...
(PA), (ii) edema
Edema
Edema or oedema ; both words from the Greek , oídēma "swelling"), formerly known as dropsy or hydropsy, is an abnormal accumulation of fluid beneath the skin or in one or more cavities of the body that produces swelling...
factor (EF), and (iii) lethal factor (LF).
Anthrax toxin is an A/B toxin
Interestingly, each individual anthrax toxin protein is, in fact, nontoxic. Toxic symptoms are not observed when these proteins are injected individually into laboratory animals. However, the co-injection of PA and EF causes edemaEdema
Edema or oedema ; both words from the Greek , oídēma "swelling"), formerly known as dropsy or hydropsy, is an abnormal accumulation of fluid beneath the skin or in one or more cavities of the body that produces swelling...
, and the co-injection of PA and LF is lethal. The former combination is called edema toxin, and the latter combination is called lethal toxin. Thus the manifestation of physiological symptoms requires PA, in either case.
The PA requirement observed in animal-model experiments demonstrates a common paradigm for bacterial toxins, called the A / B paradigm. The A component is enzymatically active, and the B component is the cell binding component. Anthrax toxin, in fact, is of the form A2B, where the two enzymes, EF and LF, are the A components and PA is the B component. Thus PA acts as a Trojan Horse
Trojan Horse
The Trojan Horse is a tale from the Trojan War about the stratagem that allowed the Greeks finally to enter the city of Troy and end the conflict. In the canonical version, after a fruitless 10-year siege, the Greeks constructed a huge wooden horse, and hid a select force of men inside...
, which carries EF and LF through the plasma membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
into the cytosol, where they may then catalyze reactions that disrupt normal cellular physiology.
Anthrax toxin assembly and translocation

Endothelium
The endothelium is the thin layer of cells that lines the interior surface of blood vessels, forming an interface between circulating blood in the lumen and the rest of the vessel wall. These cells are called endothelial cells. Endothelial cells line the entire circulatory system, from the heart...
marker-8 (TEM8
ANTXR1
Anthrax toxin receptor 1 is a protein that in humans is encoded by the ANTXR1 gene.-Further reading:...
) and capillary
Capillary
Capillaries are the smallest of a body's blood vessels and are parts of the microcirculation. They are only 1 cell thick. These microvessels, measuring 5-10 μm in diameter, connect arterioles and venules, and enable the exchange of water, oxygen, carbon dioxide, and many other nutrient and waste...
morphogenesis
Morphogenesis
Morphogenesis , is the biological process that causes an organism to develop its shape...
protein 2 (CMG2). Then a 20 kDa fragment (PA20) is cleaved off of PA83's amino terminus by membrane endoproteases from the furin family. When PA20 dissociates, the remaining receptor-bound portion of PA, called PA63, may assemble into either a heptameric or octameric ring-shaped oligomer
Oligomer
In chemistry, an oligomer is a molecule that consists of a few monomer units , in contrast to a polymer that, at least in principle, consists of an unlimited number of monomers. Dimers, trimers, and tetramers are oligomers. Many oils are oligomeric, such as liquid paraffin...
. This ring-shaped oligomer is often referred to as the pre-pore (or pre-channel) form of PA, since later in the pathway it will become a translocase pore (or channel). The surface of the pre-pore oligomer, which was exposed upon release of the PA20 moiety, can then bind to LF and EF.. The heptameric and octameric forms of the PA oligomer may then bind with up to three or four molecules of EF and/or LF, respectively. The cell then endocytoses these assembled complexes and carries them to an acidic compartment in the cell. The low pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
encountered in the endosome causes the PA63 pre-channel to convert into a cation-selective channel. EF and LF are driven through the channel by a pH gradient, allowing the enzyme factors to enter the cytosol
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
.
Enzyme function of LF and EF
Once in the cytosol, the EF and LF then carry out their respective damage-inducing processes.- EF acts as a Ca2+ and calmodulinCalmodulinCalmodulin is a calcium-binding protein expressed in all eukaryotic cells...
dependent adenylate cyclaseCyclaseA cyclase is an enzyme, almost always a lyase, that catalyzes a chemical reaction to form a cyclic compound. Important cyclase enzymes include:* Adenylyl cyclase, which forms cyclic AMP from adenosine triphosphate...
that greatly increases the level of cAMPCyclic adenosine monophosphateCyclic adenosine monophosphate is a second messenger important in many biological processes...
in the cell. This increase in cAMP upsets water homeostasisHomeostasisHomeostasis is the property of a system that regulates its internal environment and tends to maintain a stable, constant condition of properties like temperature or pH...
, severely throws the intracellular signaling pathwaysSignal transductionSignal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
off balance, and impairs macrophage function, allowing the bacteria to further evade the immune system.
- LF also helps the bacteria evade the immune system through killing macrophages. Once in these cells, LF acts as a Zn2+-dependent endoproteaseProteaseA protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
that snips off the N-terminus of mitogen-activated protein kinase kinases (MAPKK)Mitogen-activated protein kinaseMitogen-activated protein kinases are serine/threonine-specific protein kinases that respond to extracellular stimuli and regulate various cellular activities, such as gene expression, mitosis, differentiation, proliferation, and cell survival/apoptosis.-Activation:MAP kinases are activated...
. This inhibits these kinases by not allowing them to efficiently bind to their substrates, which leads to altered signaling pathways and ultimately to apoptosisApoptosisApoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
.
Thus, the synergistic effect of these three proteins leads to cellular death through a cascade of events that allow the proteins to enter the cell and disrupt cellular function.
Extracellular toxin structure-function relationship
The mechanism of anthrax toxin action is the result of the molecular structures of the three toxin proteins in combination with biomolecules of the host cell. The molecular interactions are apparent upon performing a detailed analysis of the structures of PA, EF, LF, and the cellular receptors (ANTXR1ANTXR1
Anthrax toxin receptor 1 is a protein that in humans is encoded by the ANTXR1 gene.-Further reading:...
and ANTXR2
ANTXR2
Anthrax toxin receptor 2 is a protein that in humans is encoded by the ANTXR2 gene.-Further reading:...
). Structures for the toxin molecules (Figs. 3–5),,, the receptor,, and for the complexes of the molecules all provided insight on the synergistic actions of these proteins. Analyses on binding sites and conformational changes augmented the structural studies, elucidating the functions of each domain of PA, LF, and EF, as briefly outlined in Table 1.
The structure of PA was the first to be determined (Fig. 3). This structure and that of its cellular receptor shed much light on the specificity of recognition and binding. This specificity of PA and the receptor CMG2 (similar to type I integins) is due to interactions through a metal ion dependent adhesion site (MIDAS), a hydrophobic groove, and a β-hairpin projection. These all contribute to a tight interaction in which much protein surface area on CMG2 (and TEM8) is buried.
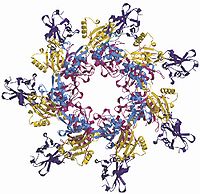
Heptamerization and pore formation is sterically hindered by the PA20 fragment, but when it is removed from the top of the monomer, the pre-pore is quickly formed. The heptamer formation causes no major changes in the conformation of each individual monomer, but by coming together, more than 15400 Ų (154 nm²) of protein surface is buried. This buried surface consists mostly of polar or charged side groups from domains 1 and 2.
During the heptamerization of PA63, molecules of EF and/or LF rapidly and simultaneously bind to the heptamer pre-pore. This binding occurs because after removing the PA20 domain, a large site is uncovered on domain 1 of PA63. Domain 1 provides a large surface that the interacts with the N-terminus of EF and LF, which is almost completely homologous for the first ~36 residues and similar in tertiary structure for the first ~250 residues. Studies on the binding region of LF and EF demonstrated that a large surface area contacts with domain 1 of two adjacent PA63 molecules when in the heptamer conformation. This large binding area explains why previous studies could only bind up to three molecules on a PA63 heptamer. The LF/EF binding site is now being utilized for delivery of therapeutics via fusion proteins.
Upon formation of the prepore and attachment of LF and/or EF, the heptamer migrates to a lipid raft where it is rapidly endocytosed. Endocytosis
Endocytosis
Endocytosis is a process by which cells absorb molecules by engulfing them. It is used by all cells of the body because most substances important to them are large polar molecules that cannot pass through the hydrophobic plasma or cell membrane...
occurs as a result of a series of events. This begins when CMG2 or TEM8 is palmitoylated, which inhibits the association of the receptor with lipid rafts. This inhibits the receptor from being endocytosed before PA83 is cleaved and before LF or EF can associate with the heptamer. Reassociation of the receptor with the cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
and glycosphigolipid-rich microdomains (lipid rafts) occurs when PA63 binds to the receptor and heptamerizes. Once the receptor and PA returns to the lipid raft, E3 ubiquitin ligase Cb1 ubiquitinates the cytoplasmic tail of the receptor, signaling the receptor and associated toxin proteins for endocytosis. Dynamin
Dynamin
Dynamin is a GTPase responsible for endocytosis in the eukaryotic cell. Dynamins are principally involved in the scission of newly formed vesicles from the membrane of one cellular compartment and their targeting to, and fusion with, another compartment, both at the cell surface as well as at the...
and Eps15 are required for this endocytosis to occur, indicating that anthrax toxin enters the cell via the clathrin
Clathrin
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1975. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice...
-dependent pathway.
As discussed, each molecule interacts with several others in order to induce the endocytosis of the anthrax toxin. Once inside, the complex is transferred to an acidic compartment, where the heptamer, still in the non-membrane-spanning pre-pore conformation, is prepared for translocation of EF and LF into the cytosol.
Pore formation
At first glance, the primary sequence of PA does not look like that of a membrane-spanning protein. A hydrophobicity plot lacking any patterns which are common to possible membrane-spanning domains. The structures of other multimeric membrane proteins (such as diphtheria toxinDiphtheria toxin
Diphtheria toxin is an exotoxin secreted by Corynebacterium diphtheriae, the pathogen bacterium that causes diphtheria. Unusually, the toxin gene is encoded by a bacteriophage...
) provide the answer to how PA manages to span the membrane. It is thought that PA acts like these multimeric membrane proteins that form β-barrels made from stretchs of both polar and non-polar amino acids from each monomer.
The formation of the β-barrel pore is facilitated with a drop in pH. To form the barrel when the pH drops, PA63 domain 2 must undergo the greatest conformation change. Upon examination of the structure of domain 2 (Fig. 7), one can see that this domain contains a Greek-key motif (the gold portion in Fig. 7). A general schematic of a Greek-key motif is shown in Fig. 8. Attached to the Greek-key in domain 2 is a large disordered loop. The necessity of this loop in pore formation is shown through using mutagenesis and proteolysis of the loop with chymotrypsin. Additional electrophysiological measurements of cysteine substitutions place the amino acids of this loop inside the lumen of the membrane inserted pore. The disordered loop in domain 2 also has a pattern of alternating hydrophobic and hydrophilic amino acids, which is a pattern conserved in the membrane-spanning portions of porins. The only problem is that the loop is not large enough to span a membrane in a β-barrel. This membrane insertion could only occur with additional conformational changes. A large conformational change takes place where the Greek-key motif unfolds, forming a β-hairpin that projects downward into the membrane and forms a β-barrel with the other 6 monomers of the complex (figures 9a and 9b). The final pore has a diameter of 12 Å (1.2 nm), which fits the theoretical value of this model.
This model would require large conformational changes in domain 2 along with the breaking of many hydrogen bonds as the Greek-key motif peels away from the center of the domain. Petosa et al. proposed a model of how this occurs. Insertion of the PA Greek key motifs into the membrane occurs when the heptamer is acidified. On artificial bilayers, this occurs when the pH is dropped from 7.4 to 6.5, suggesting that the trigger for insertion involves a titration of histidines. This indeed fits the sequence of PA since domain 2 contains a number of histidines (shown as asterisks in figure 9a). Three histidine residues are found in the disordered loop, one of which lies with a Greek-key histidine within a cluster of polar amino acids. This cluster (including the two histidines, three arginines and one glutamate) is embedded at the top of the Greek-key motif, so it is easy to see that the protonation of these histidines would disrupt the cluster. Furthermore, another histidine is located at the base of the Greek-key motif along with a number of hydrophobic residues (on the green segment in figures 7 and 9a). At pH 7.4 this segment is ordered, but when the crystals are grown at pH 6.0, it becomes disordered. This order to disorder transition is the initial step of PA membrane insertion.
PA is endocytosed as a soluble heptamer attached to its receptors, with LF or EF attached to the heptamer as cargo. The first step after endocytosis is the acidification of the endocytotic vesicle. The acidification plays two roles in the lifespan of the toxin. First, it helps to relax the tight grip of the CMG2 or TEM8 receptor on PA, facilitating the pore formation (the different receptors allow for insertion at a slightly different pH). Second, the drop in pH causes a disordered loop and a Greek-key motif in the PA domain 2 to fold out of the heptamer pre-pore and insert through the wall of the acidic vesicle, leading to pore formation (Figures 7–9).
Santelli et al. explained more about the process after they determined the crystal structure of the PA/CMG2 complex. The structure of this complex shows the binding of CMG2 by both domain 2 and 4 of PA. This interaction demonstrates less freedom to unfold the Greek key. Further analysis shows that seven of the nine histidines in PA are on the domain 2/domain 4 interface. Protonation of these histidines causes the domains to separate enough to allow the Greek-key to flop out and help form the β-hairpin involved in insertion. In addition, when PA binds to CMG2, insertion no longer occurs at a pH of 6.5, as it does when inserted into an artificial membrane. Instead it requires a pH of 5.0 for insertion in natural cells. This difference was explained to be the result of the pocket next to the MIDAS motif in CMG2. This pocket contains a histidine buried at the bottom where domain 2 attaches. This histidine is protonated at a lower pH and adds greater stability to PA. This added stability keeps the Greek-key from being able to move until more acidic conditions are met. These histidines all work in conjunction to keep the heptamer from inserting prematurely before endocytosis occurs.
Santelli and colleagues (Fig. 10) also built a hypothetical structure of the membrane-inserted PA/CMG2 structure. This model shows that the β-barrel is about 70 Å (7 nm) long, 30 Å (3 nm) of which span the membrane and the 40 Å (4 nm) gap is actually filled in with the rest of the extracellular portion of the CMG2 receptor (~100 residues). CMG2 provides additional support to the pore.
Protein translocation
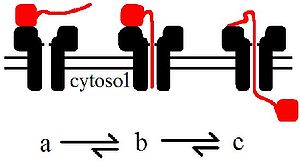
Questions for future research
Despite the recent advances in the understanding of anthrax toxin, there are still several missing details in the action of anthrax toxin. These missing details leave questions about the molecular actions inside the cell.2 What role does EF play in hindering the immune system? Does it work with LF for its effect? How do the enzymes refold after translocation? Is there a chaperoninChaperonin
Chaperonins are proteins that fold and unfold other proteins. Newly made proteins usually must fold from a linear chain of amino acids into a three-dimensional form. Chaperonins belong to a large class of molecules that assist protein folding, called molecular chaperones...
? Two proteins: Kif1C and the proteasome
Proteasome
Proteasomes are very large protein complexes inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, they are located in the nucleus and the cytoplasm. The main function of the proteasome is to degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks...
have shown a contribution to the effect of lethal toxin, but how do they contribute? Does LF target certain MAPKKs with a greater specificity? Does LF target other molecules too?

