
Alkane stereochemistry
Encyclopedia
Alkane stereochemistry concerns the stereochemistry
of alkane
s.
Alkane conformers
are one of the subjects of alkane stereochemistry.
carbon carbon sigma bond
s. The smallest alkane with such a chemical bond, ethane, exists as an infinite number of conformations with respect to rotation around the C–C bond. Two of these are recognised as energy minimum (staggered conformation) and energy maximum (eclipsed conformation) forms. The existence of specific conformations is due to hindered rotation around sigma bonds, although a role for hyperconjugation
is proposed by a competing theory.
The importance of energy minimum and energy maximum is seen by extension of these concepts to more complex molecules for which stable conformations may be predicted as minimum energy forms. The determination of stable conformations has also played a large role in the establishment of the concept of asymmetric induction
and the ability to predict the stereochemistry
of reactions controlled by steric effects.
In the example of staggered ethane
in Newman projection
, a hydrogen atom on one carbon atom has a 60° torsional angle or torsion angle with respect to the nearest hydrogen atom on the other carbon so that steric hindrance is minimised. The staggered conformation is more stable by 12.5 kJ
/mol
than the eclipsed
conformation, which is the energy maximum for ethane. In the eclipsed conformation the torsional angle is minimized.
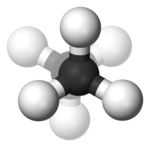
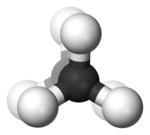
In butane
, the two staggered conformations are no longer equivalent and represent two distinct conformers:the anti-conformation (left-most, below) and the gauche conformation (right-most, below).
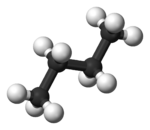
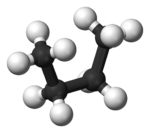
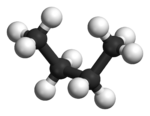
Both conformations are free of torsional strain, but, in the gauche conformation, the two methyl groups are in closer proximity than the sum of their van der Waals radii. The interaction between the two methyl groups is repulsive (van der Waals strain
), and an energy barrier results.
A measure of the potential energy
stored in butane conformers with greater steric hindrance than the 'anti'-conformer ground state is given by these values:
The eclipsed methyl group
s exert a greater steric strain because of their greater electron density
compared to lone hydrogen
atoms.
The textbook explanation for the existence of the energy maximum for an eclipsed conformation in ethane is steric hindrance, but, with a C-C bond length
of 154 pm and a Van der Waals radius
for hydrogen of 120 pm, the hydrogen atoms in ethane are never in each other's way. The question of whether steric hindrance is responsible for the eclipsed energy maximum is a topic of debate to this day. One alternative to the steric hindrance explanation is based on hyperconjugation
as analyzed within the Natural Bond Orbital framework. In the staggered conformation, one C-H sigma
bonding orbital donates electron density to the antibonding orbital of the other C-H bond. The energetic stabilization of this effect is maximized when the two orbitals have maximal overlap, occurring in the staggered conformation. There is no overlap in the eclipsed conformation, leading to a disfavored energy maximum. On the other hand, an analysis within quantitative molecular orbital theory
shows that 2-orbital-4-electron (steric) repulsions are dominant over hyperconjugation. A valence bond theory
study also emphasizes the importance of steric effects.
 Torsional strain results from resistance to twisting about a bond.
Torsional strain results from resistance to twisting about a bond.
.
Replacing hydrogen by fluorine
in polytetrafluoroethylene
changes the stereochemistry from the zigzag geometry to that of a helix
due to electrostatic repulsion of the fluorine atoms in the 1,3 positions. Evidence for the helix structure in the crystalline state is derived from X-ray crystallography
and from NMR spectroscopy
and circular dichroism
in solution.
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s.
Alkane conformers
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
are one of the subjects of alkane stereochemistry.
Conformations
Alkane conformers arise from rotation around sp3 hybridisedOrbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
carbon carbon sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
s. The smallest alkane with such a chemical bond, ethane, exists as an infinite number of conformations with respect to rotation around the C–C bond. Two of these are recognised as energy minimum (staggered conformation) and energy maximum (eclipsed conformation) forms. The existence of specific conformations is due to hindered rotation around sigma bonds, although a role for hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
is proposed by a competing theory.
The importance of energy minimum and energy maximum is seen by extension of these concepts to more complex molecules for which stable conformations may be predicted as minimum energy forms. The determination of stable conformations has also played a large role in the establishment of the concept of asymmetric induction
Asymmetric induction
Asymmetric induction in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment...
and the ability to predict the stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of reactions controlled by steric effects.
In the example of staggered ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
in Newman projection
Newman projection
A Newman projection, useful in alkane stereochemistry, visualizes chemical conformations of a carbon-carbon chemical bond from front to back, with the front carbon represented by a dot and the back carbon as a circle . The front carbon atom is called proximal, while the back atom is called distal...
, a hydrogen atom on one carbon atom has a 60° torsional angle or torsion angle with respect to the nearest hydrogen atom on the other carbon so that steric hindrance is minimised. The staggered conformation is more stable by 12.5 kJ
Joule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
than the eclipsed
Eclipsed
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X-A-B-Y is 0°. Such a conformation exists in any open chain single chemical bond connecting two sp3 hybridised atoms, and is...
conformation, which is the energy maximum for ethane. In the eclipsed conformation the torsional angle is minimized.


In butane
Butane
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or...
, the two staggered conformations are no longer equivalent and represent two distinct conformers:the anti-conformation (left-most, below) and the gauche conformation (right-most, below).



Both conformations are free of torsional strain, but, in the gauche conformation, the two methyl groups are in closer proximity than the sum of their van der Waals radii. The interaction between the two methyl groups is repulsive (van der Waals strain
Van der Waals strain
In chemistry, van der Waals strain is strain resulting from van der Waals repulsion when two substituents in a molecule approach each other with a distance less than the sum of their van der Waals radii. Van der Waals strain is also called van der Waals repulsion and is related to steric hindrance...
), and an energy barrier results.
A measure of the potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
stored in butane conformers with greater steric hindrance than the 'anti'-conformer ground state is given by these values:
- Gauche, conformer - 3.8 kJ/mol
- Eclipsed H and CH3 - 16 kJ/mol
- Eclipsed CH3 and CH3 - 19 kJ/mol.
The eclipsed methyl group
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
s exert a greater steric strain because of their greater electron density
Electron density
Electron density is the measure of the probability of an electron being present at a specific location.In molecules, regions of electron density are usually found around the atom, and its bonds...
compared to lone hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atoms.
The textbook explanation for the existence of the energy maximum for an eclipsed conformation in ethane is steric hindrance, but, with a C-C bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
of 154 pm and a Van der Waals radius
Van der Waals radius
The van der Waals radius, r, of an atom is the radius of an imaginary hard sphere which can be used to model the atom for many purposes. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms had a finite size and to...
for hydrogen of 120 pm, the hydrogen atoms in ethane are never in each other's way. The question of whether steric hindrance is responsible for the eclipsed energy maximum is a topic of debate to this day. One alternative to the steric hindrance explanation is based on hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
as analyzed within the Natural Bond Orbital framework. In the staggered conformation, one C-H sigma
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
bonding orbital donates electron density to the antibonding orbital of the other C-H bond. The energetic stabilization of this effect is maximized when the two orbitals have maximal overlap, occurring in the staggered conformation. There is no overlap in the eclipsed conformation, leading to a disfavored energy maximum. On the other hand, an analysis within quantitative molecular orbital theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
shows that 2-orbital-4-electron (steric) repulsions are dominant over hyperconjugation. A valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
study also emphasizes the importance of steric effects.
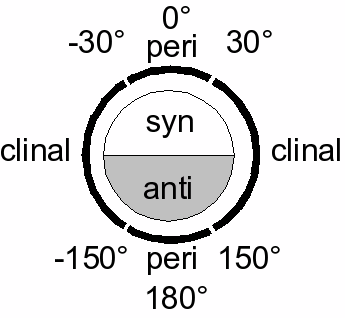
Definitions
Many definitions that describe a specific conformation (IUPAC Gold Book) exist:- a torsion angle of ±60° is called gauche
- a torsion angle between 0° and ± 90° is called syn (s)
- a torsion angle between ± 90° and 180° is called anti (a)
- a torsion angle between 30° and 150° or between –30° and –150° is called clinal
- a torsion angle between 0° and 30° or 150° and 180° is called periplanar (p)
- a torsion angle between 0° to 30° is called synperiplanar or syn- or cis-conformation (sp)
- a torsion angle between 30° to 90° and –30° to –90° is called synclinal or gauche or skew (sc)
- a torsion angle between 90° to 150°, and –90° to –150° is called anticlinal (ac)
- a torsion angle between ± 150° to 180° is called antiperiplanar or anti or trans (ap).

Special cases
In n-pentane, the terminal methyl groups experience additional pentane interferencePentane interference
Pentane interference or syn-pentane interaction is the steric hindrance that the two terminal methyl groups experience in one of the chemical conformations of n-pentane...
.
Replacing hydrogen by fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
in polytetrafluoroethylene
Polytetrafluoroethylene
Polytetrafluoroethylene is a synthetic fluoropolymer of tetrafluoroethylene that finds numerous applications. PTFE is most well known by the DuPont brand name Teflon....
changes the stereochemistry from the zigzag geometry to that of a helix
Helix
A helix is a type of smooth space curve, i.e. a curve in three-dimensional space. It has the property that the tangent line at any point makes a constant angle with a fixed line called the axis. Examples of helixes are coil springs and the handrails of spiral staircases. A "filled-in" helix – for...
due to electrostatic repulsion of the fluorine atoms in the 1,3 positions. Evidence for the helix structure in the crystalline state is derived from X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
and from NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
and circular dichroism
Circular dichroism
Circular dichroism refers to the differential absorption of left and right circularly polarized light. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral...
in solution.
See also
- More alkane conformations exist in cyclic alkanes; see cyclohexane conformationCyclohexane conformationA cyclohexane conformation is any of several three-dimensional shapes that a cyclohexane molecule can assume while maintaining the integrity of its chemical bonds....
s. - More on the impact of gauche interactions; see Gauche effect.

