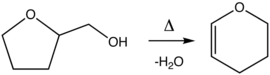
2,3-Dihydropyran
Encyclopedia
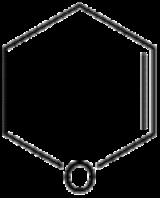
Dihydropyran is a heterocyclic compound
with the formula
C5H8O. The six-membered, not aromatic, ring has five carbon atoms and one oxygen atom. It contains one double bond. There are two isomer
s of dihydropyran that differ by the location of the double bond. 3,4-Dihydro-2H-pyran has a double bond at position 5; 3,6-dihydro-2H-pyran has the double bond at position 4.
. The numbers in front of the prefix indicate the position of the added hydrogen atoms.
The italicized capital H denotes the "indicated hydrogen", that is a second hydrogen atom present on the location where no double bond is present. The indicated hydrogen is placed just in front of the parent hydride (pyran).
Note that the position numbers of the ring-members not follow the position of the double bonds here. If also the second double bond is removed by two more hydrogen atoms we get tetrahydropyran
, or oxane.
several chemicals.
, the 2-tetrahydropyranyl group is used as a protecting group
for alcohol
s. Reaction of the alcohol with dihydropyran forms a tetrahydropyranyl ether, protecting the alcohol from a variety of reactions. The alcohol can later be restored readily by acidic hydrolysis
with formation of 5-hydroxypentanal.
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
C5H8O. The six-membered, not aromatic, ring has five carbon atoms and one oxygen atom. It contains one double bond. There are two isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s of dihydropyran that differ by the location of the double bond. 3,4-Dihydro-2H-pyran has a double bond at position 5; 3,6-dihydro-2H-pyran has the double bond at position 4.
Nomenclature
In IUPAC names, "dihydro" refers to the two added hydrogen atoms needed to remove one double bond from the parent compound pyranPyran
In chemistry, a pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ by the location of the double bonds...
. The numbers in front of the prefix indicate the position of the added hydrogen atoms.
The italicized capital H denotes the "indicated hydrogen", that is a second hydrogen atom present on the location where no double bond is present. The indicated hydrogen is placed just in front of the parent hydride (pyran).
Note that the position numbers of the ring-members not follow the position of the double bonds here. If also the second double bond is removed by two more hydrogen atoms we get tetrahydropyran
Tetrahydropyran
Tetrahydropyran is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. The compound is a colourless volatile liquid, but is obscure. Derivatives of tetrahydropyran are, however, more common. Tetrahydropyranyl ethers derived from the...
, or oxane.
3,4-Dihydropyran
3,4-Dihydropyran, also known as 2,3-dihydropyran, is used for protectingProtecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
several chemicals.
Preparation
Dihydropyran is prepared by the dehydration of tetrahydrofurfuryl alcohol over alumina at 300-400 °C.Reactions
In organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, the 2-tetrahydropyranyl group is used as a protecting group
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
for alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s. Reaction of the alcohol with dihydropyran forms a tetrahydropyranyl ether, protecting the alcohol from a variety of reactions. The alcohol can later be restored readily by acidic hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
with formation of 5-hydroxypentanal.