
Xylylene
Encyclopedia
Xylylene comprises two isomeric organic compound
s with the formula C6H4(CH2)2. These compounds are related to the corresponding quinone
s by replacement of the oxygen atoms by CH2 groups. ortho- and para
-xylylene are best known, although neither is stable in solid or liquid form. Certain substituted derivatives of xylylenes are however highly stable, an example being tetracyanoquinodimethane.
or, more readily, the α-substituted derivatives (see equation). Upon condensation, p-xylylene dimer
izes with moderate efficiency to give p-cyclophane
:
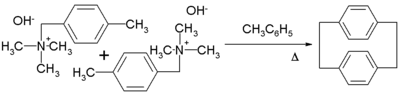 Further heating of the p-cyclophane gives poly(para-xylylene)
Further heating of the p-cyclophane gives poly(para-xylylene)
.
The reaction of α,α'-dibromo-o-xylene with iron carbonyls affords low yields of the xylylene complex Fe(CO)3[η4-C6H4(CH2)2]. This complex is similar to Fe(CO)3[η4-1,3-butadiene].
At high temperatures, benzocyclobutene
s can undergo electrocyclic ring-opening
to form o-xylylenes. This and other syntheses of o-xylylenes, and their subsequent dimerization by [4+4] cycloaddition
to form cycloctyl structures, were used repeatedly in the synthesis of superphane
.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s with the formula C6H4(CH2)2. These compounds are related to the corresponding quinone
Quinone
A quinone is a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C– groups with any necessary rearrangement of double bonds," resulting in "a fully conjugated cyclic dione structure."...
s by replacement of the oxygen atoms by CH2 groups. ortho- and para
Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.- Ortho, meta, and para substitution :...
-xylylene are best known, although neither is stable in solid or liquid form. Certain substituted derivatives of xylylenes are however highly stable, an example being tetracyanoquinodimethane.
Synthesis and reactivity
p-Xylylene forms upon pyrolysis of p-xyleneXylene
Xylene encompasses three isomers of dimethylbenzene. The isomers are distinguished by the designations ortho- , meta- , and para- , which specify to which carbon atoms the two methyl groups are attached...
or, more readily, the α-substituted derivatives (see equation). Upon condensation, p-xylylene dimer
Dimer
A dimer is a chemical entity consisting of two structurally similar subunits called monomers joined by bonds that can be either strong or weak.- Organic chemistry :...
izes with moderate efficiency to give p-cyclophane
Cyclophane
A cyclophane is a hydrocarbon consisting of an aromatic unit and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known...
:

Parylene
Parylene is the tradename for a variety of chemical vapor deposited poly polymers used as moisture and dielectric barriers. Among them, Parylene C is the most popular due to its combination of barrier properties, cost, and other processing advantages.Parylene is green polymer chemistry...
.
The reaction of α,α'-dibromo-o-xylene with iron carbonyls affords low yields of the xylylene complex Fe(CO)3[η4-C6H4(CH2)2]. This complex is similar to Fe(CO)3[η4-1,3-butadiene].
At high temperatures, benzocyclobutene
Benzocyclobutene
Benzocyclobutene is a benzene ring fused to a cyclobutane ring. It has chemical formula 88.BCB is frequently used to create photosensitive polymers. BCB-based polymer dielectrics may be spun on or applied to various substrates for use in Micro Electro-Mechanical Systems and microelectronics...
s can undergo electrocyclic ring-opening
Electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
to form o-xylylenes. This and other syntheses of o-xylylenes, and their subsequent dimerization by [4+4] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
to form cycloctyl structures, were used repeatedly in the synthesis of superphane
Superphane
[2.2.2.2.2.2]Cyclophane or superphane is a 6-fold bridged cyclophane with all arene positions in the benzene dimer taken up by ethylene spacers. The compound has been of some scientific interest as a model for testing aromaticity and was first synthesised by Boekelheide in 1979. Superphane is the...
.

