
Water-in-water emulsion
Encyclopedia
An emulsion
is two immiscible liquids mixed together (by shaking for example) with small droplets of one liquid dispersed (separated and distributed throughout the space) in the other liquid. This dispersion
is usually not stable and all the droplets will “clump” together over time and forms two layers. Because of the immiscibility, the emulsion is classified according to the chemical nature of the liquids such as oil-in-water (O/W), e.g. micelles, or water-in-oil (W/O), inverted micelles, and sometimes with complex structure such as water-in-oil-in-water (W/O/W). These classical types of emulsions can be stabilized from coalescence
(i.e. preventing the droplets from clumping together) by the presence of surfactant
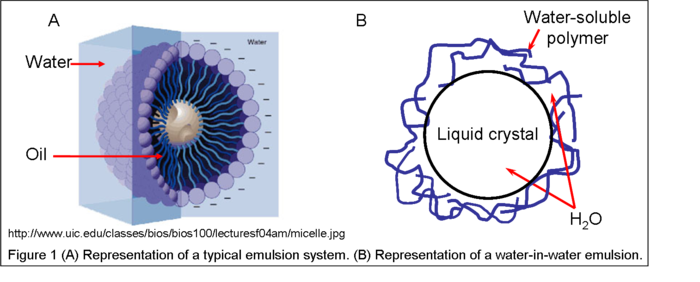
molecules, of which part of the molecular structure is soluble in water, and the other part is soluble in oil-like solvents (Fig 1A).
Theoretically, a water-in-water (W/W) emulsion is a system that consists of droplets of water-solvated molecules in another continuous aqueous solution; both the droplet and continuous phases contain different moleucles that are entirely water-soluble. As such, when two entirely aqueous solutions containing different water-soluble molecules are mixed together, water droplets containing predominantly one component are dispersed in water solution containing another component. Recently, such a water-in-water emulsion was demonstrated to exist and be stable from coalescence by the separation of different types of non-amphiphilic, but water-soluble molecular interactions. These molecular interactions include hydrogen bonding, pi stacking, and salt bridging. This w/w emulsion was generated when the different water-solvated molecular functional groups get segregated in an aqueous mixture consisting of polymer
and liquid crystal
molecules(Fig. 1B).
 This water-in-water emulsion consists of liquid crystals suspended as water-solvated droplets dispersed in a solution of polymer whose solvent is also water. The liquid crystal component of the emulsion is disodium cromolyn glycate (DSCG) (Fig. 2). This molecule is an anti-asthmatic drug, but also exists as a special type of liquid crystal when the concentration of DSCG is ~9-21 wt%. Unlike conventional lyotropic liquid crystals which consist of oily molecules such as 5CB, DSCG molecules are not amphiphilic, but entirely water-soluble. Thus, the separation of hydrophobic/hydrophilic groups cannot be applied to DSCG. The polymer solution serves as the medium or continuous phase of the w/w emulsion. Apart from being water-soluble, one important criterium for the generation of this w/w emulsion system is that the polymer cannot bear functional groups that interact strongly with DSCG. As such, ionic polymer when mixed with DSCG does not form w/w emulsion, but gives rise to a homogeneous
This water-in-water emulsion consists of liquid crystals suspended as water-solvated droplets dispersed in a solution of polymer whose solvent is also water. The liquid crystal component of the emulsion is disodium cromolyn glycate (DSCG) (Fig. 2). This molecule is an anti-asthmatic drug, but also exists as a special type of liquid crystal when the concentration of DSCG is ~9-21 wt%. Unlike conventional lyotropic liquid crystals which consist of oily molecules such as 5CB, DSCG molecules are not amphiphilic, but entirely water-soluble. Thus, the separation of hydrophobic/hydrophilic groups cannot be applied to DSCG. The polymer solution serves as the medium or continuous phase of the w/w emulsion. Apart from being water-soluble, one important criterium for the generation of this w/w emulsion system is that the polymer cannot bear functional groups that interact strongly with DSCG. As such, ionic polymer when mixed with DSCG does not form w/w emulsion, but gives rise to a homogeneous
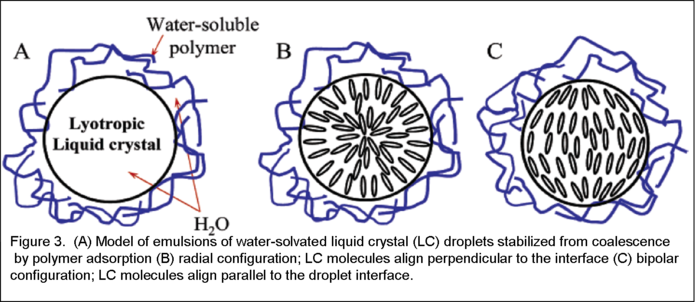
solution or a precipitate solution. Consequently, the known polymers that afford w/w emulsion include polyacrylic amides and polyols. Surprisingly, some of these water-in-water emulsions can be exceptionally stable from coalescence for up to 30 days.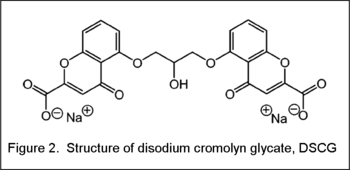 Because molecules of liquid crystal assume a preferred common orientation among themselves, the overall orientation of liquid crystals in a droplet is only stable in certain configurations (Fig. 3). As water solvated droplets in a w/w emulsion, DSCG molecules would align in a preferred direction on the surface of the droplet. In order to minimize the overall energy of the system, the DSCG molecules in the droplet prefer to align either parallel or perpendicular to the surfaces of the droplets.(Fig. 4A,B).
Because molecules of liquid crystal assume a preferred common orientation among themselves, the overall orientation of liquid crystals in a droplet is only stable in certain configurations (Fig. 3). As water solvated droplets in a w/w emulsion, DSCG molecules would align in a preferred direction on the surface of the droplet. In order to minimize the overall energy of the system, the DSCG molecules in the droplet prefer to align either parallel or perpendicular to the surfaces of the droplets.(Fig. 4A,B).
The stability of this water-in-water emulsion from coalescence is attributed to three molecular forces:
1. The separation of different molecular forces at the beginning of the droplet formation. Similar forces tend to stay together: pi-stacking and salt bridging are the two dominant forces in the liquid crystal droplet phase, while hydrogen bonding governs in the continuous polymer phase.
2. As the droplet size increases, the molecular interactions at the interface of the droplet phase and the continuous phase become stronger through multivalent
interactions. The strengthening of interfacial molecular interactions in w/w emulsions results in the formation of a layer of polymer that coats the surface of the droplet which consequently prevents droplets from clumping together.
3. In addition, it is also proposed that when two liquid crystal droplets merge together (coalescence), the orientation of the liquid crystal molecules in the two merging droplets must change to “adapt” to each other, and thus incur an energy penalty which prevent the occurrence of coalescence.
 This w/w emulsion also represents a new class of polymer dispersed liquid crystals(PDLC). Traditionally known PDLC consists of oil-in-water emulsion where the oily droplet is a thermotropic liquid crystal such as 4-pentyl-4'-cyanobiphenyl (5CB), and the water phase contains certain polymers. In comparison, this water-in-water emulsion consists of Polymer-Dispersed Lyotropic
This w/w emulsion also represents a new class of polymer dispersed liquid crystals(PDLC). Traditionally known PDLC consists of oil-in-water emulsion where the oily droplet is a thermotropic liquid crystal such as 4-pentyl-4'-cyanobiphenyl (5CB), and the water phase contains certain polymers. In comparison, this water-in-water emulsion consists of Polymer-Dispersed Lyotropic
Liquid Crystals, where the lyotropic liquid crystal is DSCG molecules solvated in water. Traditional PDLCs have found application, from switchable windows to projection displays. The water-in-water emulsion of polymer-dispersed lyotropic liquid crystals has the potential for building highly bio-functional materials because of its compatibility with protein structure.
Other known types of water-in-water emulsions involve the separation of different biopolymers in aqueous solution.


2. Tutorial on liquid crystals http://outreach.lci.kent.edu/
3. Introduction to polymer dispersed liquid crystals (PDLC)
4. Droplet configuration of PDLC’s http://plc.cwru.edu/tutorial/enhanced/files/pdlc/droplet/droplet.htm
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible . Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion is used when both the dispersed and the...
is two immiscible liquids mixed together (by shaking for example) with small droplets of one liquid dispersed (separated and distributed throughout the space) in the other liquid. This dispersion
Dispersion
Dispersion may refer to:In physics:*The dependence of wave velocity on frequency or wavelength:**Dispersion , for light waves**Dispersion **Acoustic dispersion, for sound waves...
is usually not stable and all the droplets will “clump” together over time and forms two layers. Because of the immiscibility, the emulsion is classified according to the chemical nature of the liquids such as oil-in-water (O/W), e.g. micelles, or water-in-oil (W/O), inverted micelles, and sometimes with complex structure such as water-in-oil-in-water (W/O/W). These classical types of emulsions can be stabilized from coalescence
Coalescence
Coalescence may refer to:* Coalescence , the merging of genetic lineages backwards time to a most recent common ancestor* Coalescence , the merging of two or more phonological segments into one...
(i.e. preventing the droplets from clumping together) by the presence of surfactant
Surfactant
Surfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
molecules, of which part of the molecular structure is soluble in water, and the other part is soluble in oil-like solvents (Fig 1A).
Theoretically, a water-in-water (W/W) emulsion is a system that consists of droplets of water-solvated molecules in another continuous aqueous solution; both the droplet and continuous phases contain different moleucles that are entirely water-soluble. As such, when two entirely aqueous solutions containing different water-soluble molecules are mixed together, water droplets containing predominantly one component are dispersed in water solution containing another component. Recently, such a water-in-water emulsion was demonstrated to exist and be stable from coalescence by the separation of different types of non-amphiphilic, but water-soluble molecular interactions. These molecular interactions include hydrogen bonding, pi stacking, and salt bridging. This w/w emulsion was generated when the different water-solvated molecular functional groups get segregated in an aqueous mixture consisting of polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
and liquid crystal
Liquid crystal
Liquid crystals are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. For instance, an LC may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of LC phases, which can be...
molecules(Fig. 1B).

Homogeneous (chemistry)
A substance that is uniform in composition is a definition of homogeneous. This is in contrast to a substance that is heterogeneous.The definition of homogeneous strongly depends on the context used. In Chemistry, a homogeneous suspension of material means that when dividing the volume in half, the...
solution or a precipitate solution. Consequently, the known polymers that afford w/w emulsion include polyacrylic amides and polyols. Surprisingly, some of these water-in-water emulsions can be exceptionally stable from coalescence for up to 30 days.

The stability of this water-in-water emulsion from coalescence is attributed to three molecular forces:
1. The separation of different molecular forces at the beginning of the droplet formation. Similar forces tend to stay together: pi-stacking and salt bridging are the two dominant forces in the liquid crystal droplet phase, while hydrogen bonding governs in the continuous polymer phase.
2. As the droplet size increases, the molecular interactions at the interface of the droplet phase and the continuous phase become stronger through multivalent
Multivalent
Multivalent may refer to:*Multivalent , the property of having multiple valences*Polyvalent: something which has many values, meanings, or appeals*Multivalent , a Java-based web browser...
interactions. The strengthening of interfacial molecular interactions in w/w emulsions results in the formation of a layer of polymer that coats the surface of the droplet which consequently prevents droplets from clumping together.
3. In addition, it is also proposed that when two liquid crystal droplets merge together (coalescence), the orientation of the liquid crystal molecules in the two merging droplets must change to “adapt” to each other, and thus incur an energy penalty which prevent the occurrence of coalescence.

Lyotropic
A material is called lyotropic if it forms liquid crystal phases because of the addition of a solvent. Historically the term was used to describe materials composed of amphiphilic molecules. Such molecules comprise a water-loving 'hydrophilic' head-group attached to a water-hating 'hydrophobic'...
Liquid Crystals, where the lyotropic liquid crystal is DSCG molecules solvated in water. Traditional PDLCs have found application, from switchable windows to projection displays. The water-in-water emulsion of polymer-dispersed lyotropic liquid crystals has the potential for building highly bio-functional materials because of its compatibility with protein structure.
Other known types of water-in-water emulsions involve the separation of different biopolymers in aqueous solution.


External links
1. Salt bridging and example of salt bridges http://www.cryst.bbk.ac.uk/PPS2/projects/day/TDayDiss/SaltBridges.html2. Tutorial on liquid crystals http://outreach.lci.kent.edu/
3. Introduction to polymer dispersed liquid crystals (PDLC)
- http://plc.cwru.edu/tutorial/enhanced/files/pdlc/intro/intro.htm
4. Droplet configuration of PDLC’s http://plc.cwru.edu/tutorial/enhanced/files/pdlc/droplet/droplet.htm

