
Virial expansion
Encyclopedia
The classical virial expansion expresses the pressure
of a many-particle system in equilibrium
as a power series in the density
.
The virial expansion was introduced in 1901 by Heike Kamerlingh Onnes
as a generalization of the ideal gas
law. He wrote for a gas containing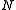 atoms
atoms
or molecules,
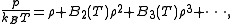
where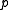 is the pressure,
is the pressure, 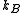 is the Boltzmann constant,
is the Boltzmann constant, 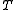 the absolute temperature, and
the absolute temperature, and
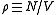 is the number density of the
is the number density of the
gas.
Note that for a gas containing a fraction of
of 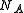 (Avogadro's number) molecules, truncation of the virial expansion after the
(Avogadro's number) molecules, truncation of the virial expansion after the
first term leads to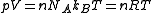 , which is the ideal gas
, which is the ideal gas
law.
Writing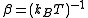 , the virial expansion can be written in closed form as
, the virial expansion can be written in closed form as
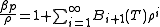 .
.
The virial coefficient
s are characteristic of the interactions between the particles in the system and in general depend on the temperature
are characteristic of the interactions between the particles in the system and in general depend on the temperature  .
.
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
of a many-particle system in equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
as a power series in the density
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
.
The virial expansion was introduced in 1901 by Heike Kamerlingh Onnes
Heike Kamerlingh Onnes
Heike Kamerlingh Onnes was a Dutch physicist and Nobel laureate. He pioneered refrigeration techniques, and he explored how materials behaved when cooled to nearly absolute zero. He was the first to liquify helium...
as a generalization of the ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
law. He wrote for a gas containing
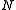 atoms
atomsor molecules,
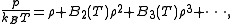
where
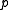 is the pressure,
is the pressure, 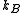 is the Boltzmann constant,
is the Boltzmann constant, 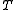 the absolute temperature, and
the absolute temperature, and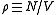 is the number density of the
is the number density of thegas.
Note that for a gas containing a fraction
 of
of 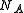 (Avogadro's number) molecules, truncation of the virial expansion after the
(Avogadro's number) molecules, truncation of the virial expansion after thefirst term leads to
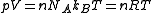 , which is the ideal gas
, which is the ideal gasIdeal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
law.
Writing
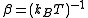 , the virial expansion can be written in closed form as
, the virial expansion can be written in closed form as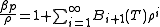 .
.The virial coefficient
Virial coefficient
Virial coefficients B_i appear as coefficients in the virial expansion of the pressure of a many-particle system in powers of the density, providing systematic corrections to the ideal gas law...
s
 are characteristic of the interactions between the particles in the system and in general depend on the temperature
are characteristic of the interactions between the particles in the system and in general depend on the temperature  .
.

