
Vapor quality
Encyclopedia
In thermodynamics
, vapor quality is a quantitative description of the usefulness of a vapor
to do mechanical work
. The quality of a fluid is the percentage of mass
that is vapor
; i.e. saturated vapor has a "quality" of 100%, and saturated liquid has a "quality" of 0%. For instance, in analysis of the Rankine cycle
the quality of a multi-phase working fluid
would be understood to imply this definition.
Vapor quality is an intensive property which can be used in conjunction with another independent intensive properties to specify the thermodynamic state
of a thermodynamic system
. It has no meaning for substances which are not saturated mixtures (i.e., compressed liquids or superheated vapors).
Quality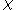 can be calculated by dividing the mass of the vapor by the mass of the total mixture:
can be calculated by dividing the mass of the vapor by the mass of the total mixture: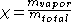
where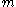 indicates mass.
indicates mass.
Another definition used by chemical engineers defines quality (q) of a fluid as the fraction that is saturated liquid. By this definition, a saturated liquid has q = 1. A saturated vapor has q = 0.
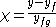 ,
,
where is equal to either specific enthalpy, specific entropy
is equal to either specific enthalpy, specific entropy
, specific volume
or specific internal energy,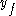 is the value of the specific property of the substance in the liquid state while under saturated conditions, and
is the value of the specific property of the substance in the liquid state while under saturated conditions, and 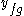 is the value of the specific property of the substance in the gas state minus that of the liquid state.
is the value of the specific property of the substance in the gas state minus that of the liquid state.
Another expression of the same concept is: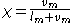
where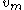 is the vapor mass and
is the vapor mass and 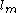 is the liquid mass.
is the liquid mass.
, where an important application was the steam engine. Low quality steam would contain a high moisture
percentage and therefore damage components more easily. High quality steam would not corrode the steam engine. Steam engine
s use water vapor (steam
) to drive pistons which create work. The quality of steam can be quantitatively described by steam quality (steam dryness), the proportion of saturated
steam
in a saturated water/steam mixture. i.e., a steam quality of 0 indicates 100% water while a steam quality of 1 (or 100%) indicates 100% steam.
The quality of steam on which steam whistle
s are blown is variable and may affect frequency
. Steam quality determines the velocity of sound
, which declines with decreasing dryness due to the inertia
of the liquid phase. Also, the specific volume
of steam for a given temperature decreases with decreasing dryness.
Steam quality is very useful in determining enthalpy
of saturated water/steam mixtures since the enthalpy of steam (gaseous state) is many orders of magnitude higher than enthalpy of water (liquid state).
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, vapor quality is a quantitative description of the usefulness of a vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
to do mechanical work
Mechanical work
In physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work...
. The quality of a fluid is the percentage of mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
that is vapor
Vapor
A vapor or vapour is a substance in the gas phase at a temperature lower than its critical point....
; i.e. saturated vapor has a "quality" of 100%, and saturated liquid has a "quality" of 0%. For instance, in analysis of the Rankine cycle
Rankine cycle
The Rankine cycle is a cycle that converts heat into work. The heat is supplied externally to a closed loop, which usually uses water. This cycle generates about 90% of all electric power used throughout the world, including virtually all solar thermal, biomass, coal and nuclear power plants. It is...
the quality of a multi-phase working fluid
Working fluid
A working fluid is a pressurized gas or liquid that actuates a machine. Examples include steam in a steam engine, air in a hot air engine and hydraulic fluid in a hydraulic motor or hydraulic cylinder...
would be understood to imply this definition.
Vapor quality is an intensive property which can be used in conjunction with another independent intensive properties to specify the thermodynamic state
Thermodynamic state
A thermodynamic state is a set of values of properties of a thermodynamic system that must be specified to reproduce the system. The individual parameters are known as state variables, state parameters or thermodynamic variables. Once a sufficient set of thermodynamic variables have been...
of a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
. It has no meaning for substances which are not saturated mixtures (i.e., compressed liquids or superheated vapors).
Quality
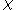 can be calculated by dividing the mass of the vapor by the mass of the total mixture:
can be calculated by dividing the mass of the vapor by the mass of the total mixture: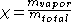
where
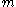 indicates mass.
indicates mass.Another definition used by chemical engineers defines quality (q) of a fluid as the fraction that is saturated liquid. By this definition, a saturated liquid has q = 1. A saturated vapor has q = 0.
Calculation
The above expression for vapor quality can be expressed as: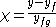 ,
,where
 is equal to either specific enthalpy, specific entropy
is equal to either specific enthalpy, specific entropyEntropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
, specific volume
Specific volume
In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass. It is the reciprocal of density:In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass...
or specific internal energy,
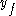 is the value of the specific property of the substance in the liquid state while under saturated conditions, and
is the value of the specific property of the substance in the liquid state while under saturated conditions, and 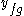 is the value of the specific property of the substance in the gas state minus that of the liquid state.
is the value of the specific property of the substance in the gas state minus that of the liquid state.Another expression of the same concept is:
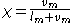
where
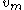 is the vapor mass and
is the vapor mass and 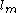 is the liquid mass.
is the liquid mass.Steam quality
The genesis of the idea of vapor quality was derived from the origins of thermodynamicsThermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, where an important application was the steam engine. Low quality steam would contain a high moisture
Moisture
Humidity is the amount of moisture the air can hold before it rains. Moisture refers to the presence of a liquid, especially water, often in trace amounts...
percentage and therefore damage components more easily. High quality steam would not corrode the steam engine. Steam engine
Steam engine
A steam engine is a heat engine that performs mechanical work using steam as its working fluid.Steam engines are external combustion engines, where the working fluid is separate from the combustion products. Non-combustion heat sources such as solar power, nuclear power or geothermal energy may be...
s use water vapor (steam
Steam
Steam is the technical term for water vapor, the gaseous phase of water, which is formed when water boils. In common language it is often used to refer to the visible mist of water droplets formed as this water vapor condenses in the presence of cooler air...
) to drive pistons which create work. The quality of steam can be quantitatively described by steam quality (steam dryness), the proportion of saturated
Saturation (chemistry)
In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
steam
Steam
Steam is the technical term for water vapor, the gaseous phase of water, which is formed when water boils. In common language it is often used to refer to the visible mist of water droplets formed as this water vapor condenses in the presence of cooler air...
in a saturated water/steam mixture. i.e., a steam quality of 0 indicates 100% water while a steam quality of 1 (or 100%) indicates 100% steam.
The quality of steam on which steam whistle
Steam whistle
A steam whistle is a device used to produce sound with the aid of live steam, which acts as a vibrating system .- Operation :...
s are blown is variable and may affect frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
. Steam quality determines the velocity of sound
Velocity of Sound
Velocity of Sound is an album by The Apples in Stereo. It was the group's fifth album, released in October 2002. The American release has an orange album cover, while the European version is green and the Japanese version is blue...
, which declines with decreasing dryness due to the inertia
Inertia
Inertia is the resistance of any physical object to a change in its state of motion or rest, or the tendency of an object to resist any change in its motion. It is proportional to an object's mass. The principle of inertia is one of the fundamental principles of classical physics which are used to...
of the liquid phase. Also, the specific volume
Specific volume
In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass. It is the reciprocal of density:In thermodynamics, the specific volume of a substance is the ratio of the substance's volume to its mass...
of steam for a given temperature decreases with decreasing dryness.
Steam quality is very useful in determining enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
of saturated water/steam mixtures since the enthalpy of steam (gaseous state) is many orders of magnitude higher than enthalpy of water (liquid state).

