
Urea carboxylase
Encyclopedia
In enzymology, an urea carboxylase is an enzyme
that catalyzes
the chemical reaction
The 3 substrates
of this enzyme are ATP
, urea
, and HCO3-, whereas its 3 products
are ADP
, phosphate
, and urea-1-carboxylate (allophanate).
This enzyme belongs to the family of ligase
s, specifically those forming generic carbon-nitrogen bonds. The systematic name of this enzyme class is urea:carbon-dioxide ligase (ADP-forming). Other names in common use include urease (ATP-hydrolysing), urea carboxylase (hydrolysing), ATP-urea amidolyase, urea amidolyase, UALase, and UCA. This enzyme participates in urea cycle and metabolism of amino groups. It employs one cofactor
, biotin
.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- ATP + urea + HCO3-
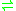 ADP + phosphate + urea-1-carboxylate
ADP + phosphate + urea-1-carboxylate
The 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
, and HCO3-, whereas its 3 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
, phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
, and urea-1-carboxylate (allophanate).
This enzyme belongs to the family of ligase
Ligase
In biochemistry, ligase is an enzyme that can catalyse the joining of two large molecules by forming a new chemical bond, usually with accompanying hydrolysis of a small chemical group dependent to one of the larger molecules...
s, specifically those forming generic carbon-nitrogen bonds. The systematic name of this enzyme class is urea:carbon-dioxide ligase (ADP-forming). Other names in common use include urease (ATP-hydrolysing), urea carboxylase (hydrolysing), ATP-urea amidolyase, urea amidolyase, UALase, and UCA. This enzyme participates in urea cycle and metabolism of amino groups. It employs one cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
, biotin
Biotin
Biotin, also known as Vitamin H or Coenzyme R, is a water-soluble B-complex vitamin discovered by Bateman in 1916. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring...
.

