
Unbinilium
Encyclopedia
Unbinilium also called eka
-radium
or element 120, is the temporary, systematic element name
of a hypothetical chemical element
in the periodic table
that has the temporary symbol Ubn and has the atomic number
120.
Since unbinilium is placed below the alkaline earth metals it possibly has properties similar to those of radium or barium
.
Attempts to date to synthesize the element using fusion reactions at low excitation energy have met with failure, although there are reports that the fission of nuclei of unbinilium at very high excitation has been successfully measured, indicating a strong shell effect at Z=120.
in Dubna
by bombarding a plutonium
-244 target with iron
-58 ion
s. Initial analysis revealed that no atoms of element 120 were produced providing a limit of 400 fb
for the cross section at the energy studied.
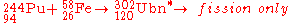
The Russian team are planning to upgrade their facilities before attempting the reaction again.
In April 2007, the team at GSI
attempted to create unbinilium using uranium
-238 and nickel
-64:

No atoms were detected providing a limit of 1.6 pb on the cross section at the energy provided. The GSI repeated the experiment with higher sensitivity in three separate runs from April–May 2007, Jan–March 2008, and Sept–Oct 2008, all with negative results and providing a cross section limit of 90 fb.
, with the compound nucleus 302Ubn being the most stable of those that can be created directly by current methods. It has been calculated that Z=120 may in fact be the next proton magic number, rather than at Z=114 or 126.
Several experiments have been performed between 2000–2008 at the Flerov laboratory of Nuclear Reactions in Dubna studying the fission characteristics of the compound nucleus 302Ubn. Two nuclear reactions have been used, namely 244Pu+58Fe and 238U+64Ni. The results have revealed how nuclei such as this fission predominantly by expelling closed shell nuclei such as 132Sn (Z=50, N=82). It was also found that the yield for the fusion-fission pathway was similar between 48Ca and 58Fe projectiles, indicating a possible future use of 58Fe projectiles in superheavy element formation.
In 2008, the team at GANIL, France, described the results from a new technique which attempts to measure the fission half-life
of a compound nucleus at high excitation energy, since the yields are significantly higher than from neutron evaporation channels. It is also a useful method for probing the effects of shell closures on the survivability of compound nuclei in the super-heavy region, which can indicate the exact position of the next proton shell (Z=114, 120, 124, or 126).
The team studied the nuclear fusion reaction between uranium ions and a target of natural nickel:
The results indicated that nuclei of unbinilium were produced at high (~70 MeV) excitation energy which underwent fission with measurable half-lives > 10−18s. Although very short, the ability to measure such a process indicates a strong shell effect at Z=120. At lower excitation energy (see neutron evaporation), the effect of the shell will be enhanced and ground-state nuclei can be expected to have relatively long half-lives. This result could partially explain the relatively long half-life of 294Uuo
measured in experiments at Dubna. Similar experiments have indicated a similar phenomenon at Z=124 (see unbiquadium
) but not for ununquadium
, suggesting that the next proton shell does in fact lie at Z>120.
Likewise, the team at RIKEN have also begun a program utilizing 248Cm targets and have indicated future experiments to probe the possibility of Z=120 being the next magic number using the aforementioned nuclear reactions to form 302Ubn.
half-lives of several isotope
s of unbinilium (namely, 292-304Ubn) have been predicted to be around 1–20 microseconds.
and highly explosive in terms of flammability.
It is also possible that, due to relativistic effects
, the element has noble gas
character, which may be also the case for ununquadium
. A predicted oxidation state
is II.
MD = multi-dimensional; DNS = dinuclear system; AS = advanced statistical; σ = cross section
Mendeleev's predicted elements
Professor Dmitri Mendeleev published the first Periodic Table of the Atomic Elements in 1869 based on properties which appeared with some regularity as he laid out the elements from lightest to heaviest....
-radium
Radium
Radium is a chemical element with atomic number 88, represented by the symbol Ra. Radium is an almost pure-white alkaline earth metal, but it readily oxidizes on exposure to air, becoming black in color. All isotopes of radium are highly radioactive, with the most stable isotope being radium-226,...
or element 120, is the temporary, systematic element name
Systematic element name
A systematic element name is the temporary name and symbol assigned to newly synthesized and not yet synthesized chemical elements. In chemistry, a transuranic element receives a permanent name and symbol only after its synthesis has been confirmed. In some cases, this has been a protracted and...
of a hypothetical chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
that has the temporary symbol Ubn and has the atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
120.
Since unbinilium is placed below the alkaline earth metals it possibly has properties similar to those of radium or barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
.
Attempts to date to synthesize the element using fusion reactions at low excitation energy have met with failure, although there are reports that the fission of nuclei of unbinilium at very high excitation has been successfully measured, indicating a strong shell effect at Z=120.
Neutron evaporation
In March–April 2007, the synthesis of unbinilium was attempted at the Flerov Laboratory of Nuclear ReactionsJoint Institute for Nuclear Research
The Joint Institute for Nuclear Research, JINR , in Dubna, Moscow Oblast , Russia, is an international research centre for nuclear sciences, with 5500 staff members, 1200 researchers including 1000 Ph.D.s from eighteen member states The Joint Institute for Nuclear Research, JINR , in Dubna, Moscow...
in Dubna
Dubna
Dubna is a town in Moscow Oblast, Russia. It has a status of naukograd , being home to the Joint Institute for Nuclear Research, an international nuclear physics research centre and one of the largest scientific foundations in the country. It is also home to MKB Raduga, a defence aerospace company...
by bombarding a plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
-244 target with iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
-58 ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s. Initial analysis revealed that no atoms of element 120 were produced providing a limit of 400 fb
Barn (unit)
A barn is a unit of area. Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is used in all fields of high energy physics to express the cross sections of any scattering process, and is best understood as a measure of the...
for the cross section at the energy studied.
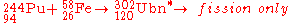
The Russian team are planning to upgrade their facilities before attempting the reaction again.
In April 2007, the team at GSI
Gesellschaft für Schwerionenforschung
The GSI Helmholtz Centre for Heavy Ion Research GmbH in the Wixhausen suburb of Darmstadt, Germany is a federally and state co-funded heavy ion research center. The current director of GSI is Horst Stöcker who succeeded Walter F...
attempted to create unbinilium using uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
-238 and nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
-64:

No atoms were detected providing a limit of 1.6 pb on the cross section at the energy provided. The GSI repeated the experiment with higher sensitivity in three separate runs from April–May 2007, Jan–March 2008, and Sept–Oct 2008, all with negative results and providing a cross section limit of 90 fb.
Compound nucleus fission
Unbinilium is of interest because it is part of the hypothesized island of stabilityIsland of stability
The island of stability in nuclear physics describes a set of as-yet undiscovered isotopes of transuranium elements which are theorized to be much more stable than others...
, with the compound nucleus 302Ubn being the most stable of those that can be created directly by current methods. It has been calculated that Z=120 may in fact be the next proton magic number, rather than at Z=114 or 126.
Several experiments have been performed between 2000–2008 at the Flerov laboratory of Nuclear Reactions in Dubna studying the fission characteristics of the compound nucleus 302Ubn. Two nuclear reactions have been used, namely 244Pu+58Fe and 238U+64Ni. The results have revealed how nuclei such as this fission predominantly by expelling closed shell nuclei such as 132Sn (Z=50, N=82). It was also found that the yield for the fusion-fission pathway was similar between 48Ca and 58Fe projectiles, indicating a possible future use of 58Fe projectiles in superheavy element formation.
In 2008, the team at GANIL, France, described the results from a new technique which attempts to measure the fission half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of a compound nucleus at high excitation energy, since the yields are significantly higher than from neutron evaporation channels. It is also a useful method for probing the effects of shell closures on the survivability of compound nuclei in the super-heavy region, which can indicate the exact position of the next proton shell (Z=114, 120, 124, or 126).
The team studied the nuclear fusion reaction between uranium ions and a target of natural nickel:
The results indicated that nuclei of unbinilium were produced at high (~70 MeV) excitation energy which underwent fission with measurable half-lives > 10−18s. Although very short, the ability to measure such a process indicates a strong shell effect at Z=120. At lower excitation energy (see neutron evaporation), the effect of the shell will be enhanced and ground-state nuclei can be expected to have relatively long half-lives. This result could partially explain the relatively long half-life of 294Uuo
Ununoctium
Ununoctium is the temporary IUPAC name for the transactinide element having the atomic number 118 and temporary element symbol Uuo. It is also known as eka-radon or element 118, and on the periodic table of the elements it is a p-block element and the last one of the 7th period. Ununoctium is...
measured in experiments at Dubna. Similar experiments have indicated a similar phenomenon at Z=124 (see unbiquadium
Unbiquadium
Unbiquadium , also known as eka-uranium or element 124, is the temporary name of a hypothetical element in the periodic table that has the temporary symbol Ubq and atomic number 124....
) but not for ununquadium
Ununquadium
Ununquadium is the temporary name of a radioactive chemical element with the temporary symbol Uuq and atomic number 114. There is no proposed name yet, although flerovium has been discussed in the media.About 80 decays of atoms of...
, suggesting that the next proton shell does in fact lie at Z>120.
Future reactions
The GSI have plans to start up a program utilizing 248Cm targets for superheavy element production and will most likely attempt this reaction in 2011.Likewise, the team at RIKEN have also begun a program utilizing 248Cm targets and have indicated future experiments to probe the possibility of Z=120 being the next magic number using the aforementioned nuclear reactions to form 302Ubn.
Calculated decay characteristics
In a quantum tunneling model with mass estimates from a macroscopic-microscopic model, the alpha-decayAlpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
half-lives of several isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of unbinilium (namely, 292-304Ubn) have been predicted to be around 1–20 microseconds.
Extrapolated reactivity
Unbinilium should be highly reactive, according to periodic trends, as this element is a member of alkaline earth metals. It would be much more reactive than any other lighter elements of this group. If group reactivity is followed, this element would react violently in air to form an oxide (UbnO) and in water to form the hydroxide, which would be a strong baseBase (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
and highly explosive in terms of flammability.
It is also possible that, due to relativistic effects
Relativistic quantum chemistry
Relativistic quantum chemistry invokes quantum chemical and relativistic mechanical arguments to explain elemental properties and structure, especially for heavy elements of the periodic table....
, the element has noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
character, which may be also the case for ununquadium
Ununquadium
Ununquadium is the temporary name of a radioactive chemical element with the temporary symbol Uuq and atomic number 114. There is no proposed name yet, although flerovium has been discussed in the media.About 80 decays of atoms of...
. A predicted oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
is II.
Target-projectile combinations leading to Z=120 compound nuclei
The below table contains various combinations of targets and projectiles which could be used to form compound nuclei with an atomic number of 120.| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 232Th | 70Zn | 302Ubn | |
| 238U | 64Ni | 302Ubn | |
| 244Pu | 58Fe | 302Ubn | |
| 248Cm | 54Cr | 302Ubn | |
| 249Cf | 50Ti | 299Ubn | |
| 257Fm | 48Ca | 305Ubn |
Theoretical calculations on evaporation cross sections
The below table contains various targets-projectile combinations for which calculations have provided estimates for cross section yields from various neutron evaporation channels. The channel with the highest expected yield is given.MD = multi-dimensional; DNS = dinuclear system; AS = advanced statistical; σ = cross section
| Target | Projectile | CN | Channel (product) | ~σmax | Model | Ref |
|---|---|---|---|---|---|---|
| 208Pb | 88Sr | 296Ubn | 1n (295Ubn) | 70 fb | DNS | |
| 208Pb | 87Sr | 295Ubn | 1n (294Ubn) | 80 fb | DNS | |
| 208Pb | 88Sr | 296Ubn | 1n (295Ubn) | <0.1 fb | MD | |
| 238U | 64Ni | 302Ubn | 3n (299Ubn) | 3 fb | MD | |
| 238U | 64Ni | 302Ubn | 2n (300Ubn) | 0.5 fb | DNS | |
| 238U | 64Ni | 302Ubn | 4n (298Ubn) | 2 ab | DNS-AS | |
| 244Pu | 58Fe | 302Ubn | 4n (298Ubn) | 5 fb | MD | |
| 244Pu | 58Fe | 302Ubn | 3n (299Ubn) | 8 fb | DNS-AS | |
| 248Cm | 54Cr | 302Ubn | 3n (299Ubn) | 10 pb | DNS-AS | |
| 248Cm | 54Cr | 302Ubn | 4n (298Ubn) | 30 fb | MD | |
| 249Cf | 50Ti | 299Ubn | 4n (295Ubn) | 45 fb | MD | |
| 249Cf | 50Ti | 299Ubn | 3n (296Ubn) | 40 fb | MD | |
| 257Fm | 48Ca | 305Ubn | 3n (302Ubn) | 70 fb | DNS |


