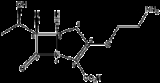
Thienamycin
Encyclopedia
Thienamycin, one of the most potent naturally produced antibiotics known thus far, was discovered in Streptomyces cattleya in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase
enzyme
s.
Thienamycin is a zwitterion
at pH 7.
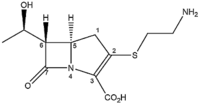 Thienamycin was the first among the naturally occurring class of carbapenem
Thienamycin was the first among the naturally occurring class of carbapenem
antibiotics to be discovered and isolated. Carbapenems are similar in structure to their antibiotic “cousins” the penicillins. Like penicillins, carbapenems contain a β-lactam ring (cyclic amide) fused to a five-membered ring. Carbapenems differ in structure from penicillins in that within the five-membered ring a sulfur is replaced by a carbon atom (C1) and an unsaturation is present between C2 and C3 in the five-membered ring.
,Staphylococcus epidermidis
, Pseudomonas aeruginosa
to name a few). In a study carried out by Spratt et al., they found that, although thienamycin binds to all of the penicillin-binding proteins (PBP) in Escherichia coli
, it preferentially binds to PBP-1 and PBP-2, which are both associated with the elongation of the cell wall.
Unlike penicillins, which are rendered ineffective through rapid hydrolysis by the β-lactamase enzyme present in some strains of bacteria, thienamycin remains antimicrobially active. Neu et al. found that thienamycin displayed high activity against bacteria that were resistant to other β-lactamase stable compounds (cephalosporins), highlighting the superiority of thienamycin as an antibiotic
among β-lactams.
, -valine
, and -α-aminoadipic acid
by ACV synthetase (ACVS, a nonribsomal peptide synthetase) and then cyclization of this formed tripeptide by isopenicillin N synthetase (IPNS).
The gene cluster (thn) for the biosynthesis of thienamycin of S. cattleya was identified and sequenced in 2003, lending insight into the biosynthetic mechanism for thienamycin formation. The biosynthesis is thought to share features with the biosynthesis of the simple carbapenem
s, beginning with the condensation of malonyl-CoA
with glutamate-5-semialdehyde to form the pyrroline
ring. The β-lactam is then formed by a β-lactam synthetase, which makes use of ATP
, providing a carbapenam
. At some later point, oxidation to the carbapenem
and ring inversions must occur.
The hydroxyethyl side chain of thienamycin is thought to be a result of two separate methyl transfers from S-adenosyl methionine
. According to the proposed gene functions, ThnK, ThnL, and ThnP could catalyze these methyl-transfer steps. A β-lactam synthetase (ThnM) is thought to catalyze the formation of the β-lactam ring fused to the five-membered ring. How the cysteaminyl side-chain is incorporated is largely unknown, although ThnT, ThnR, and ThnH are involved in the processing of CoA to cysteamine for use in the pathway. Various oxidations complete the biosynthesis.
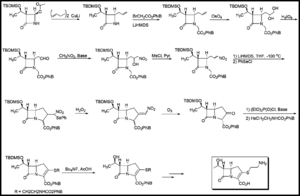
 Due to low titre and to difficulties in isolating and purifying thienamycin produced via fermentation, total synthesis is the preferred method for commercial production. Numerous methods are available in the literature for the total synthesis of thienamycin. One synthetic route is given in Figure 3.
Due to low titre and to difficulties in isolating and purifying thienamycin produced via fermentation, total synthesis is the preferred method for commercial production. Numerous methods are available in the literature for the total synthesis of thienamycin. One synthetic route is given in Figure 3.
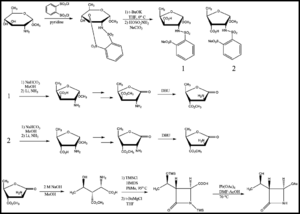
The starting β-lactam for the pathway given above can be synthesized via the following method (Figure 4):
— was formulated in 1985. Imipenem, an N-formimidoyl derivative of thienamycin, is rapidly metabolized by a renal dihpeptidase enzyme found in the human body. To prevent its rapid degradation, imipenem is normally co-administered with cilastatin
, an inhibitor of this enzyme.
Beta-lactamase
Beta-lactamases are enzymes produced by some bacteria and are responsible for their resistance to beta-lactam antibiotics like penicillins, cephamycins, and carbapenems . These antibiotics have a common element in their molecular structure: a four-atom ring known as a beta-lactam...
enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s.
Thienamycin is a zwitterion
Zwitterion
In chemistry, a zwitterion is a neutral molecule with a positive and a negative electrical charge at different locations within that molecule. Zwitterions are sometimes also called inner salts.-Examples:...
at pH 7.
History
In 1976, fermentation broths obtained from the soil bacteria Streptomyces cattleya were found to be active in screens for inhibitors of peptidoglycan biosynthesis. Initial attempts to isolate the active species proved difficult due to the chemical instability of that component. After many attempts and extensive purification, the material was finally isolated in >90% purity, allowing for the structural elucidation of thienamycin in 1979 (Figure 1).
Carbapenem
Carbapenems are a class of β-lactam antibiotics with a broad spectrum of antibacterial activity. They have a structure that renders them highly resistant to most β-lactamases...
antibiotics to be discovered and isolated. Carbapenems are similar in structure to their antibiotic “cousins” the penicillins. Like penicillins, carbapenems contain a β-lactam ring (cyclic amide) fused to a five-membered ring. Carbapenems differ in structure from penicillins in that within the five-membered ring a sulfur is replaced by a carbon atom (C1) and an unsaturation is present between C2 and C3 in the five-membered ring.
Mechanism of Action
In vitro, thienamycin employs a similar mode of action as penicillins through disrupting the cell wall synthesis (peptidoglycan biosynthesis) of various Gram-positive and Gram-negative bacteria (Staphylococcus aureusStaphylococcus aureus
Staphylococcus aureus is a facultative anaerobic Gram-positive coccal bacterium. It is frequently found as part of the normal skin flora on the skin and nasal passages. It is estimated that 20% of the human population are long-term carriers of S. aureus. S. aureus is the most common species of...
,Staphylococcus epidermidis
Staphylococcus epidermidis
Staphylococcus epidermidis is one of thirty-three known species belonging to the genus Staphylococcus. It is part of human skin flora, and consequently part of human flora. It can also be found in the mucous membranes and in animals. Due to contamination, it is probably the most common species...
, Pseudomonas aeruginosa
Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common bacterium that can cause disease in animals, including humans. It is found in soil, water, skin flora, and most man-made environments throughout the world. It thrives not only in normal atmospheres, but also in hypoxic atmospheres, and has, thus, colonized many...
to name a few). In a study carried out by Spratt et al., they found that, although thienamycin binds to all of the penicillin-binding proteins (PBP) in Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
, it preferentially binds to PBP-1 and PBP-2, which are both associated with the elongation of the cell wall.
Unlike penicillins, which are rendered ineffective through rapid hydrolysis by the β-lactamase enzyme present in some strains of bacteria, thienamycin remains antimicrobially active. Neu et al. found that thienamycin displayed high activity against bacteria that were resistant to other β-lactamase stable compounds (cephalosporins), highlighting the superiority of thienamycin as an antibiotic
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
among β-lactams.
Biosynthesis
The formation of thienamycin is thought to occur through a different pathway from classic β-lactams (penicillins, cephalosporins). Production of classic β-lactams in both fungi and bacteria occur through two steps: First, the condensation of -cysteineCysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
, -valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
, and -α-aminoadipic acid
Adipic acid
Adipic acid is the organic compound with the formula 42. From the industrial perspective, it is the most important dicarboxylic acid: About 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon...
by ACV synthetase (ACVS, a nonribsomal peptide synthetase) and then cyclization of this formed tripeptide by isopenicillin N synthetase (IPNS).
The gene cluster (thn) for the biosynthesis of thienamycin of S. cattleya was identified and sequenced in 2003, lending insight into the biosynthetic mechanism for thienamycin formation. The biosynthesis is thought to share features with the biosynthesis of the simple carbapenem
Carbapenem
Carbapenems are a class of β-lactam antibiotics with a broad spectrum of antibacterial activity. They have a structure that renders them highly resistant to most β-lactamases...
s, beginning with the condensation of malonyl-CoA
Malonyl-CoA
Malonyl-CoA is a coenzyme A derivative.-Functions:It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis....
with glutamate-5-semialdehyde to form the pyrroline
Pyrroline
Pyrrolines, also known under the name dihydropyrroles, are three different heterocyclic organic chemical compounds that differ in the position of the double bond. Pyrrolines are formally derived from the aromate pyrrole by hydrogenation...
ring. The β-lactam is then formed by a β-lactam synthetase, which makes use of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, providing a carbapenam
Carbapenam
A carbapenam is a β-lactam compound that is a saturated carbapenem. They exist primarily as biosynthetic intermediates on the way to the carbapenem antibiotics....
. At some later point, oxidation to the carbapenem
Carbapenem
Carbapenems are a class of β-lactam antibiotics with a broad spectrum of antibacterial activity. They have a structure that renders them highly resistant to most β-lactamases...
and ring inversions must occur.
The hydroxyethyl side chain of thienamycin is thought to be a result of two separate methyl transfers from S-adenosyl methionine
S-Adenosyl methionine
S-Adenosyl methionine is a common cosubstrate involved in methyl group transfers. SAM was first discovered in Italy by G. L. Cantoni in 1952. It is made from adenosine triphosphate and methionine by methionine adenosyltransferase . Transmethylation, transsulfuration, and aminopropylation are the...
. According to the proposed gene functions, ThnK, ThnL, and ThnP could catalyze these methyl-transfer steps. A β-lactam synthetase (ThnM) is thought to catalyze the formation of the β-lactam ring fused to the five-membered ring. How the cysteaminyl side-chain is incorporated is largely unknown, although ThnT, ThnR, and ThnH are involved in the processing of CoA to cysteamine for use in the pathway. Various oxidations complete the biosynthesis.
Total Synthesis


The starting β-lactam for the pathway given above can be synthesized via the following method (Figure 4):
Clinical Use
Thienamycin itself is extremely unstable and decomposes in aqueous solution. Consequently, thienamycin is impractical for the clinical treatment of bacterial infections. For this reason, stable derivatives of thienamycin were created for medicinal consumption. One such derivative — imipenemImipenem
Imipenem is an intravenous β-lactam antibiotic developed in 1980. It has an extremely broad spectrum of activity.Imipenem belongs to the subgroup of carbapenems. It is derived from a compound called thienamycin, which is produced by the bacterium Streptomyces cattleya...
— was formulated in 1985. Imipenem, an N-formimidoyl derivative of thienamycin, is rapidly metabolized by a renal dihpeptidase enzyme found in the human body. To prevent its rapid degradation, imipenem is normally co-administered with cilastatin
Cilastatin
Cilastatin is a chemical compound which inhibits the human enzyme dehydropeptidase.Dehydropeptidase is found in the kidney and is responsible for degrading the antibiotic imipenem. Cilastatin is therefore combined intravenously with imipenem in order to protect it from dehydropeptidase and prolong...
, an inhibitor of this enzyme.

