
Surface conductivity
Encyclopedia
Surface conductivity is an additional conductivity
of an electrolyte
in the vicinity of charged surfaces. Close to charged surfaces a layer of counter ions of opposite polarity exists which is attracted by the surface charge
s. This layer of higher ionic concentration is a part of the interfacial double layer
. The concentration of the ions in this layer is higher as compared to the volume conductivity
far from the charged surface and leads to a higher conductivity of this layer.
Smoluchowski
was the first to recognize the importance of surface conductivity at the beginning of the 20th century.
There is a detailed description of surface conductivity by Lyklema in "Fundamentals of Interface and Colloid Science"
The Double Layer (DL) has two regions, according to the well established Gouy-Chapman-Stern model, Ref.2. The upper level, which is in contact with the bulk fluid is the diffuse layer. The inner layer that is in contact with interface is the Stern layer.
It is possible that the lateral motion of ions in both parts of the DL contributes to the surface conductivity.
The contribution of the Stern layer is less well described. It is often called "additional surface conductivity".
The theory of the surface conductivity of the diffuse part of the DL was developed by Bikerman. He derived a simple equation that links surface conductivity κσ with the behaviour of ions at the interface. For symmetrical electrolyte and assuming identical ions diffusion coefficients D+=D-=D it is given in Ref.2:
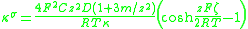
where
The parameter m characterizes the contribution of electro-osmosis
to the motion of ions within the DL: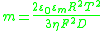
The Dukhin number
is a dimensionless parameter that characterizes the contribution of the surface conductivity to a variety of electrokinetic phenomena
, such as, electrophoresis
and electroacoustic phenomena
.
Electrical resistivity and conductivity
Electrical resistivity is a measure of how strongly a material opposes the flow of electric current. A low resistivity indicates a material that readily allows the movement of electric charge. The SI unit of electrical resistivity is the ohm metre...
of an electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
in the vicinity of charged surfaces. Close to charged surfaces a layer of counter ions of opposite polarity exists which is attracted by the surface charge
Surface charge
Surface charge is the electric charge present at an interface. There are many different processes which can lead to a surface being charged, including adsorption of ions, protonation/deprotonation, and the application of an external electric field...
s. This layer of higher ionic concentration is a part of the interfacial double layer
Double layer (interfacial)
A double layer is a structure that appears on the surface of an object when it is placed into a liquid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object...
. The concentration of the ions in this layer is higher as compared to the volume conductivity
Conductivity (electrolytic)
The conductivity of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens per meter ....
far from the charged surface and leads to a higher conductivity of this layer.
Smoluchowski
Marian Smoluchowski
Marian Smoluchowski was an ethnic Polish scientist in the Austro-Hungarian Empire. He was a pioneer of statistical physics and an avid mountaineer.-Life:...
was the first to recognize the importance of surface conductivity at the beginning of the 20th century.
There is a detailed description of surface conductivity by Lyklema in "Fundamentals of Interface and Colloid Science"
The Double Layer (DL) has two regions, according to the well established Gouy-Chapman-Stern model, Ref.2. The upper level, which is in contact with the bulk fluid is the diffuse layer. The inner layer that is in contact with interface is the Stern layer.
It is possible that the lateral motion of ions in both parts of the DL contributes to the surface conductivity.
The contribution of the Stern layer is less well described. It is often called "additional surface conductivity".
The theory of the surface conductivity of the diffuse part of the DL was developed by Bikerman. He derived a simple equation that links surface conductivity κσ with the behaviour of ions at the interface. For symmetrical electrolyte and assuming identical ions diffusion coefficients D+=D-=D it is given in Ref.2:
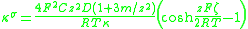
where
- F is the Faraday constant
- T is the absolute temperature
- R is the gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
- C is the ionic concentration in the bulk fluid
- z is the ionIonAn ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
valencyValence (chemistry)In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds... - ζ is the electrokinetic potential
The parameter m characterizes the contribution of electro-osmosis
Electro-osmosis
Electroosmotic flow is the motion of liquid induced by an applied potential across a porous material, capillary tube, membrane, microchannel, or any other fluid conduit...
to the motion of ions within the DL:
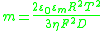
The Dukhin number
Dukhin number
Dukhin number is a dimensionless quantity that characterizes the contribution of the surface conductivity to various electrokinetic and electroacoustic effects, as well as to electrical conductivity and permittivity of fluid heterogeneous systems....
is a dimensionless parameter that characterizes the contribution of the surface conductivity to a variety of electrokinetic phenomena
Electrokinetic phenomena
Electrokinetic phenomena are a family of several different effects that occur in heterogeneous fluids or in porous bodies filled with fluid. The term heterogeneous here means a fluid containing particles...
, such as, electrophoresis
Electrophoresis
Electrophoresis, also called cataphoresis, is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. This electrokinetic phenomenon was observed for the first time in 1807 by Reuss , who noticed that the application of a constant electric...
and electroacoustic phenomena
Electroacoustic phenomena
Electroacoustic phenomena arise when ultrasound propagates through a fluid containing ions. The associated particle motion generates electric signals because ions have electric charge. This coupling between ultrasound and electric field is called electroacoustic phenomena. Fluid might be a simple...
.

