
Souders-Brown equation
Encyclopedia
Velocity
In physics, velocity is speed in a given direction. Speed describes only how fast an object is moving, whereas velocity gives both the speed and direction of the object's motion. To have a constant velocity, an object must have a constant speed and motion in a constant direction. Constant ...
in vapor–liquid separation vessels (variously called flash drums, knockout drums, knockout pots, compressor
Gas compressor
A gas compressor is a mechanical device that increases the pressure of a gas by reducing its volume.Compressors are similar to pumps: both increase the pressure on a fluid and both can transport the fluid through a pipe. As gases are compressible, the compressor also reduces the volume of a gas...
suction drums and compressor inlet drums). It has also been used for the same purpose in designing trayed fractionating column
Fractionating column
A fractionating column or fractionation column is an essential item used in the distillation of liquid mixtures so as to separate the mixture into its component parts, or fractions, based on the differences in their volatilities...
s, trayed absorption columns and other vapor–liquid-contacting columns.
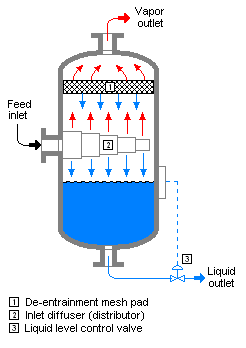
A vapor–liquid separator drum is a vertical vessel into which a liquid and vapor mixture (or a flashing liquid) is fed and wherein the liquid is separated by gravity, falls to the bottom of the vessel, and is withdrawn. The vapor travels upward at a design velocity which minimizes the entrainment
Entrainment (engineering)
Entrainment as commonly used in various branches of engineering may be defined as the entrapment of one substance by another substance. For example:* The entrapment of liquid droplets or solid particulates in a flowing gas, as with smoke....
of any liquid droplets in the vapor as it exits the top of the vessel.
Using the Souders–Brown equation
The diameter of a vapor–liquid separator drum is dictated by the expected volumetric flow rate of vapor and liquid from the drum. The following sizing methodology is based on the assumption that those flow rates are known.Use a vertical pressure vessel
Pressure vessel
A pressure vessel is a closed container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.The pressure differential is dangerous and many fatal accidents have occurred in the history of their development and operation. Consequently, their design,...
with a length–diameter ratio of about 3 to 4, and size the vessel to provide about 5 minutes of liquid inventory between the normal liquid level and the bottom of the vessel (with the normal liquid level being somewhat below the feed inlet).
Calculate the maximum allowable vapor velocity in the vessel by using the Souders–Brown equation:
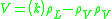
| where: | |
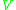 |
= maximum allowable vapor velocity, m/s |
|---|---|
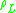 |
= liquid density, kg/m³ |
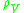 |
= vapor density, kg/m³ |
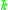 |
= 0.107 m/s (when the drum includes a de-entraining mesh pad) |
Then the cross-sectional area of the drum (A) is obtained from:
A, in m² = (vapor flow rate, in m³/s) ÷ (vapor velocity V, in m/s)
And the drum diameter (D) is:
D, in m = [ (4) (A) ÷ (3.1416) ] 0. 5
The drum should have a vapor outlet at the top, liquid outlet at the bottom, and feed inlet at about the half-full level. At the vapor outlet, provide a de-entraining mesh pad within the drum such that the vapor must pass through that mesh before it can leave the drum. Depending upon how much liquid flow is expected, the liquid outlet line should probably have a liquid level control valve.
As for the mechanical design of the drum (materials of construction, wall thickness, corrosion allowance, etc.), use the same criteria as for any pressure vessel.
Recommended values of k
The GPSA Engineering Data Book recommends the following k values for vertical drums with horizontal mesh pads (at the denoted operating pressures):- At a gauge pressure of 0 barBar (unit)The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar...
: 0.107 m/s - At a gauge pressure of 7 bar: 0.107 m/s
- At a gauge pressure of 21 bar: 0.101 m/s
- At a gauge pressure of 42 bar: 0.092 m/s
- At a gauge pressure of 63 bar: 0.083 m/s
- At a gauge pressure of 105 bar: 0.065 m/s
GPSA Notes:
- k = 0.107 at a gauge pressure of 7 bar. Subtract 0.003 for every 7 bar above a gauge pressure of 7 bar.
- For glycol or amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
solutions, multiply above k values by 0.6 – 0.8 - Typically use one-half of the above k values for approximate sizing of vertical separators without mesh pads
- For compressor suction scrubbers and expander inlet separators, multiply k by 0.7 – 0.8
External links
- High-Capacity Gas De-Entrainment (scroll to page 10 of the brochure)
- Demisters (scroll to pages 5–6 of the brochure)
- Experimental Characterization of High-Pressure Natural Gas Scrubbers by Trond Austrheim (PhD Thesis at the University of BergenUniversity of BergenThe University of Bergen is located in Bergen, Norway. Although founded as late as 1946, academic activity had taken place at Bergen Museum as far back as 1825. The university today serves more than 14,500 students...
, NorwayNorwayNorway , officially the Kingdom of Norway, is a Nordic unitary constitutional monarchy whose territory comprises the western portion of the Scandinavian Peninsula, Jan Mayen, and the Arctic archipelago of Svalbard and Bouvet Island. Norway has a total area of and a population of about 4.9 million...
, 2006)

