Skraup reaction
Encyclopedia
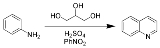
The Skraup synthesis is a chemical reaction
used to synthesize quinoline
s. It is named after the Czech chemist Zdenko Hans Skraup
(1850-1910). In the archetypal Skraup, aniline
is heated with sulfuric acid
, glycerol
, and an oxidizing agent
, like nitrobenzene
to yield quinoline.
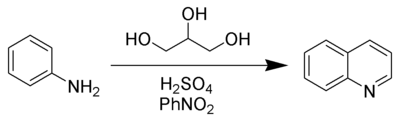 In this example, nitrobenzene
In this example, nitrobenzene
serves as both the solvent
and the oxidizing agent. The reaction, which otherwise has a reputation for being violent ("the Chemical Inquisition"), is typically conducted in the presence of ferrous sulfate
. Arsenic acid
may be used instead of nitrobenzene and the former is better since the reaction is less violent.
is unclear, yet there is good reason to believe that acrolein
(obtained by dehydration of glycerol in presence of concentrated sulfuric acid) is an intermediate, which then undergoes 1,4-addition. Acrolein itself is not used since it undergoes polymerisations under the conditions of the experiment.

Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
used to synthesize quinoline
Quinoline
Quinoline is a heterocyclic aromatic organic compound. It has the formula C9H7N and is a colourless hygroscopic liquid with a strong odour. Aged samples, if exposed to light, become yellow and later brown...
s. It is named after the Czech chemist Zdenko Hans Skraup
Zdenko Hans Skraup
Zdenko Hans Skraup was a Czech Austrian chemist who discovered the Skraup reaction, the first quinoline synthesis.-Life:...
(1850-1910). In the archetypal Skraup, aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
is heated with sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
, and an oxidizing agent
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
, like nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
to yield quinoline.

Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
serves as both the solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
and the oxidizing agent. The reaction, which otherwise has a reputation for being violent ("the Chemical Inquisition"), is typically conducted in the presence of ferrous sulfate
Iron(II) sulfate
Iron sulfate or ferrous sulfate is the chemical compound with the formula FeSO4. Known since ancient times as copperas and as green vitriol, the blue-green heptahydrate is the most common form of this material...
. Arsenic acid
Arsenic acid
Arsenic acid is the chemical compound with the formula H3AsO4. More descriptively written as AsO3, this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as such has not been isolated, but only found in solution where it...
may be used instead of nitrobenzene and the former is better since the reaction is less violent.
Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is unclear, yet there is good reason to believe that acrolein
Acrolein
Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity...
(obtained by dehydration of glycerol in presence of concentrated sulfuric acid) is an intermediate, which then undergoes 1,4-addition. Acrolein itself is not used since it undergoes polymerisations under the conditions of the experiment.


