
SNARE (protein)
Encyclopedia

The primary role of SNARE proteins is to mediate vesicle fusion
Vesicle fusion
Vesicle fusion is the merging of a vesicle with other vesicles or a part of a cell membrane. In the latter case, it is the end stage of secretion from secretory vesicles, where their contents are expelled from the cell through exocytosis at the porosome...
, that is, the exocytosis
Exocytosis
Exocytosis , also known as 'The peni-cytosis', is the durable process by which a cell directs the contents of secretory vesicles out of the cell membrane...
of cellular transport vesicles
Vesicle (biology)
A vesicle is a bubble of liquid within another liquid, a supramolecular assembly made up of many different molecules. More technically, a vesicle is a small membrane-enclosed sack that can store or transport substances. Vesicles can form naturally because of the properties of lipid membranes , or...
with the cell membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
at the porosome
Porosome
Porosomes or fusion pores are cup-shaped structures in the cell membranes of eukaryotic cells where vesicles dock in the process of vesicle fusion and secretion. These structures are about 150 nanometers in diameter and contain many different types of protein, especially SNARE proteins that mediate...
or with a target compartment (such as a lysosome
Lysosome
thumb|350px|Schematic of typical animal cell, showing subcellular components. [[Organelle]]s: [[nucleoli]] [[cell nucleus|nucleus]] [[ribosomes]] [[vesicle |vesicle]] rough [[endoplasmic reticulum]]...
).
SNAREs can be divided into two categories: vesicle or v-SNAREs , which are incorporated into the membranes of transport vesicles during budding, and target or t-SNAREs, which are located in the membranes of target compartments.
Recent classification however takes account of the structural features of the SNARE proteins and divides them into R-SNAREs and Q-SNAREs.
The best-studied SNAREs are those that mediate docking of synaptic vesicle
Synaptic vesicle
In a neuron, synaptic vesicles store various neurotransmitters that are released at the synapse. The release is regulated by a voltage-dependent calcium channel. Vesicles are essential for propagating nerve impulses between neurons and are constantly recreated by the cell...
s with the presynaptic membrane. These SNAREs are the targets of the bacterial neurotoxins responsible for botulism
Botulism
Botulism also known as botulinus intoxication is a rare but serious paralytic illness caused by botulinum toxin which is metabolic waste produced under anaerobic conditions by the bacterium Clostridium botulinum, and affecting a wide range of mammals, birds and fish...
and tetanus
Tetanus
Tetanus is a medical condition characterized by a prolonged contraction of skeletal muscle fibers. The primary symptoms are caused by tetanospasmin, a neurotoxin produced by the Gram-positive, rod-shaped, obligate anaerobic bacterium Clostridium tetani...
.
SNAREs are small, abundant and mostly plasma membrane-bound proteins. Although they vary considerably in structure and size, all share a segment in their cytosol
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
ic domain called a SNARE motif that consists of 60-70 amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s that are capable of reversible assembly into tight, four-helix bundles called "trans"-SNARE complexes.
The readily-formed metastable "trans" complexes are composed of three SNAREs: syntaxin 1 and SNAP-25
SNAP-25
Synaptosomal-associated protein 25 is a protein that in humans is encoded by the SNAP25 gene. The SNAP-25 protein is a component of the SNARE complex, which is proposed to account for the specificity of membrane fusion and to directly execute fusion by forming a tight complex that brings the...
resident in cell membrane and synaptobrevin
Synaptobrevin
Synaptobrevins are small integral membrane proteins of secretory vesicles with molecular weight of 18 kilodalton that are part of the vesicle-associated membrane protein family....
(also referred to as vesicle-associated membrane protein or VAMP) anchored in the vesicular membrane.
In neuron
Neuron
A neuron is an electrically excitable cell that processes and transmits information by electrical and chemical signaling. Chemical signaling occurs via synapses, specialized connections with other cells. Neurons connect to each other to form networks. Neurons are the core components of the nervous...
al exocytosis
Exocytosis
Exocytosis , also known as 'The peni-cytosis', is the durable process by which a cell directs the contents of secretory vesicles out of the cell membrane...
, syntaxin
Syntaxin
Syntaxins are a family of membrane integrated Q-SNARE proteins participating in exocytosis.- Domains :Syntaxins possess a single C-terminal transmembrane domain, a SNARE domain , and an N-terminal regulatory domain ....
and synaptobrevin
Synaptobrevin
Synaptobrevins are small integral membrane proteins of secretory vesicles with molecular weight of 18 kilodalton that are part of the vesicle-associated membrane protein family....
are anchored in respective membranes by their C-terminal domains, whereas SNAP-25
SNAP-25
Synaptosomal-associated protein 25 is a protein that in humans is encoded by the SNAP25 gene. The SNAP-25 protein is a component of the SNARE complex, which is proposed to account for the specificity of membrane fusion and to directly execute fusion by forming a tight complex that brings the...
is tethered to the plasma membrane via several cysteine-linked palmitoyl chains. The core SNARE complex is a four-
 -helix bundle, where one
-helix bundle, where one  -helix is contributed by syntaxin-1, one
-helix is contributed by syntaxin-1, one  -helix by synaptobrevin and two
-helix by synaptobrevin and two  -helices are contributed by SNAP-25.
-helices are contributed by SNAP-25.The plasma membrane-resident SNAREs have been shown to be present in distinct microdomains or clusters, the integrity of which is essential for the exocytotic competence of the cell.
SNARE complexes
"Trans"-SNARE complexes are protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
complexes composed of three SNARE proteins anchored in opposing (or trans
Trans
Trans is a Latin noun or prefix, meaning "across", "beyond" or "on the opposite side".Trans may refer to:- Science and technology :* Cis-trans isomerism, in chemistry, a form of stereoisomerism...
) membranes prior to membrane fusion. During fusion, the membranes merge and SNARE proteins involved in complex formation after fusion are then referred to as a "cis"-SNARE complex, because they now reside in a single (or cis
Cis
Cis may have the following meanings:* "Cis-" as a prefix of Latin origin, meaning "on the same side [as]" or "on this side [of]", with several derived usages:** In chemistry, cis- refers to cis-trans isomerism...
) resultant membrane.
R-SNAREs
R-SNAREs are proteins that contribute an arginineArginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
(R) residue in the formation of the zero ionic layer in the assembled core SNARE complex. One particular R-SNARE is synaptobrevin
Synaptobrevin
Synaptobrevins are small integral membrane proteins of secretory vesicles with molecular weight of 18 kilodalton that are part of the vesicle-associated membrane protein family....
, which is located in the synaptic vesicles.
Q-SNAREs
Q-SNAREs are proteins that contribute a glutamineGlutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
(Q) residue in the formation of the zero ionic layer in the assembled core SNARE complex. Q-SNAREs include syntaxin
Syntaxin
Syntaxins are a family of membrane integrated Q-SNARE proteins participating in exocytosis.- Domains :Syntaxins possess a single C-terminal transmembrane domain, a SNARE domain , and an N-terminal regulatory domain ....
and SNAP-25
SNAP-25
Synaptosomal-associated protein 25 is a protein that in humans is encoded by the SNAP25 gene. The SNAP-25 protein is a component of the SNARE complex, which is proposed to account for the specificity of membrane fusion and to directly execute fusion by forming a tight complex that brings the...
.
Other components
The core SNARE complex is a 4- -helix bundle. Synaptobrevin and syntaxin contribute one
-helix bundle. Synaptobrevin and syntaxin contribute one  -helix each, while SNAP-25 participates with two
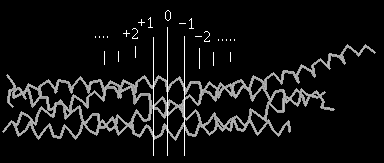
-helix each, while SNAP-25 participates with two  -helices (abbreviated as Sn1 and Sn2). The interacting amino acid residues that zip the SNARE complex can be grouped into layers. Each layer has 4 amino acid residues - one residue per each of the 4
-helices (abbreviated as Sn1 and Sn2). The interacting amino acid residues that zip the SNARE complex can be grouped into layers. Each layer has 4 amino acid residues - one residue per each of the 4  -helices. In the center of the complex is the zero ionic layer composed of one arginine (R) and three glutamine (Q) residues , and it is flanked by leucine zippering. Layers '-1', '+1' and '+2' at the centre of the complex most closely follow ideal leucine-zipper geometry and aminoacid composition.
-helices. In the center of the complex is the zero ionic layer composed of one arginine (R) and three glutamine (Q) residues , and it is flanked by leucine zippering. Layers '-1', '+1' and '+2' at the centre of the complex most closely follow ideal leucine-zipper geometry and aminoacid composition.The zero ionic layer is composed of R56 from VAMP-2, Q226 from syntaxin-1A, Q53 from Sn1 and Q174 from Sn2, and is completely buried within the leucine-zipper layers. The positively charged guanidino group of the arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
(R) residue interact with the carboxyl groups of each of the three glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
(Q) residues.
The flanking leucine-zipper layers act as a water-tight seal to shield the ionic interactions
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
from the surrounding solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
. Exposure of the zero ionic layer to the water solvent by breaking the flanking leucine zipper leads to instability of the SNARE complex and is the putative mechanism by which
 -SNAP and NSF
-SNAP and NSFN-ethylmaleimide sensitive fusion protein
N-ethylmaleimide-sensitive factor, also known as NSF or N-ethylmaleimide sensitive fusion proteins, is an enzyme which in humans is encoded by the NSF gene.- Function :...
recycle the SNARE complexes after the completion of synaptic vesicle
Synaptic vesicle
In a neuron, synaptic vesicles store various neurotransmitters that are released at the synapse. The release is regulated by a voltage-dependent calcium channel. Vesicles are essential for propagating nerve impulses between neurons and are constantly recreated by the cell...
exocytosis
Exocytosis
Exocytosis , also known as 'The peni-cytosis', is the durable process by which a cell directs the contents of secretory vesicles out of the cell membrane...
.
Proposed mechanism of membrane fusion
Assembly of the SNAREs into the "trans" complexes likely bridges the opposing lipid bilayers of membranes belonging to cell and secretory granule, bringing them in proximity and inducing their fusion. The influx of calcium into the cell triggers the completion of the assembly reaction, which is mediated by an interaction between the putative calcium sensor, synaptotagminSynaptotagmin
Synaptotagmins constitute a family of membrane-trafficking proteins that are characterized by an N-terminal transmembrane region , a variable linker, and two C-terminal C2 domains - C2A and C2B. There are 15 members in the mammalian synaptotagmin family...
, with membrane lipids and/or the partially assembled
SNARE complex.
According to the "zipper" hypothesis, the complex assembly starts at the N-terminal parts of SNARE motifs and proceeds towards the C-termini that anchor interacting proteins in membranes. Formation of the "trans"-SNARE complex proceeds through an intermediate complex composed of SNAP-25 and syntaxin-1, which later accommodates synaptobrevin-2 (the quoted syntaxin and synaptobrevin isotypes participate in neuronal neuromediator release).
Based on the stability of the resultant cis-SNARE complex, it has been postulated that energy released during the assembly process serves as a means for overcoming the repulsive forces between the membranes. There are several models that propose explanation of a subsequent step – the formation of stalk and fusion pore
Porosome
Porosomes or fusion pores are cup-shaped structures in the cell membranes of eukaryotic cells where vesicles dock in the process of vesicle fusion and secretion. These structures are about 150 nanometers in diameter and contain many different types of protein, especially SNARE proteins that mediate...
, but the exact nature of these processes remains debated. A recent in vitro single-molecule content-mixing study showed that yeast SNARE complex is enough to expand fusion pores.

