
Remineralisation
Encyclopedia
In biogeochemistry
, remineralisation (UK spelling; US remineralization) refers to the transformation of organic molecules to inorganic forms, typically mediated by biological activity.
Usually remineralisation relates to organic and inorganic molecules involving biologically important elements such as carbon
, nitrogen
and phosphorus
. For example, the following simplified equation shows the complete remineralisation of organic material with a standard Redfield ratio
to oxidised inorganic minerals such as carbon dioxide
, nitrate
(nitric acid) and phosphate
(phosphoric acid).
(C106H124O36) (NH3)16 (H3PO4) + 150 O2 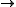 106 CO2 + 16 HNO3 + H3PO4 + 78 H2O + energy
106 CO2 + 16 HNO3 + H3PO4 + 78 H2O + energy
In reality, such complete remineralisation is likely to involve several stages each involving different organism
s and metabolic pathway
s. For example, in the case of nitrogen, its transformation from ammonia
(NH3) in the equation above, to nitrate involves the process of nitrification
, usually mediated by a series of bacteria.
Biogeochemistry
Biogeochemistry is the scientific discipline that involves the study of the chemical, physical, geological, and biological processes and reactions that govern the composition of the natural environment...
, remineralisation (UK spelling; US remineralization) refers to the transformation of organic molecules to inorganic forms, typically mediated by biological activity.
Usually remineralisation relates to organic and inorganic molecules involving biologically important elements such as carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
. For example, the following simplified equation shows the complete remineralisation of organic material with a standard Redfield ratio
Redfield ratio
Redfield ratio or Redfield stoichiometry is the molecular ratio of carbon, nitrogen and phosphorus in plankton. This empirically developed stoichiometric ratio is found to be C:N:P = 106:16:1. This term is named after the American oceanographer Alfred C. Redfield, who first described this ratio in...
to oxidised inorganic minerals such as carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
(nitric acid) and phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
(phosphoric acid).
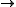 106 CO2 + 16 HNO3 + H3PO4 + 78 H2O + energy
106 CO2 + 16 HNO3 + H3PO4 + 78 H2O + energy In reality, such complete remineralisation is likely to involve several stages each involving different organism
Organism
In biology, an organism is any contiguous living system . In at least some form, all organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homoeostasis as a stable whole.An organism may either be unicellular or, as in the case of humans, comprise...
s and metabolic pathway
Metabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
s. For example, in the case of nitrogen, its transformation from ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
(NH3) in the equation above, to nitrate involves the process of nitrification
Nitrification
Nitrification is the biological oxidation of ammonia with oxygen into nitrite followed by the oxidation of these nitrites into nitrates. Degradation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle in soil...
, usually mediated by a series of bacteria.
See also
- Biological pumpBiological pumpIn oceanic biogeochemistry, the biological pump is the sum of a suite of biologically-mediated processes that transport carbon from the surface euphotic zone to the ocean's interior.-Overview:...
- DecompositionDecompositionDecomposition is the process by which organic material is broken down into simpler forms of matter. The process is essential for recycling the finite matter that occupies physical space in the biome. Bodies of living organisms begin to decompose shortly after death...
- f-ratioF-ratioIn oceanic biogeochemistry, the f-ratio is the fraction of total primary production fuelled by nitrate . This fraction is significant because it is assumed to be directly related to the sinking flux of organic marine snow from the surface ocean by the biological pump...
- John D. HamakerJohn D. HamakerJohn D. Hamaker , was an American mechanical engineer, ecologist, agronomist and science writer in the fields of soil remineralization, rock dusting, mineral cycles, climate cycles and glaciology.-Biography:Background...
(soil remineralisation) - MineralizationMineralizationMineralization may refer to:* Mineralization , the process through which an organic substance becomes impregnated by inorganic substances...

