
Quantum defect
Encyclopedia
The term quantum defect is ambiguous
. Various meanings are discussed below. Characteristic is that the defect deals with the loss on the smallest energy scale of light: that of the quantum
.
Quantum defect in laser science
In laser
science, the term quantum defect refers to the fact that the energy of a pump photon is generally higher than that of a signal photon (photon of the output radiation). The difference of energies goes to the heat;
this heat may carry away the excess of entropy
delivered with the multimode uncoherent pump.
The quantum defect of a laser
can be defined as part of the energy of the pumping photon, which is lost (not turned into photons at the lasing wavelength) in the gain medium at the lasing.
At given frequency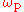 of pump
of pump
and given frequency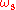 of lasing, the quantum defect
of lasing, the quantum defect 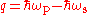 .
.
Such quantum defect has dimension of energy; for the efficient operation, the temperature
of the gain medium
(measured in units of energy) should be small compared to the quantum defect.
At a fixed pump frequency, the higher the quantum defect, the lower is the upper bound for the power efficiency.
refers to a correction applied to the equations governing Rydberg atom behavior to take into account the fact that the inner electrons do not entirely screen their associated charge in the nucleus. It is used particularity for the alkalis that contain a single electron in their outer shell.
The perfect 1/r potential in the hydrogen atom
leads to an electron
binding energy
given by
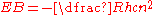 ,
,
where R is the Rydberg constant
, h is Planck's constant, c is the speed of light and n is the principal quantum number
.
For multi-electron atoms in Rydberg states with a low value of the orbital angular momentum, there is a high probability of finding the excited electron near the nucleus where it can polarize
or even penetrate the ion core, modifying the potential. The resulting shift of the energy levels is represented mathematically as an angular momentum dependent quantum defect, δl:
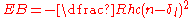 .
.
The largest shifts occur when the orbital angular momentum is equal to 0 (normally labelled 's') and these are shown in the table for the alkali metals:
Ambiguity
Ambiguity of words or phrases is the ability to express more than one interpretation. It is distinct from vagueness, which is a statement about the lack of precision contained or available in the information.Context may play a role in resolving ambiguity...
. Various meanings are discussed below. Characteristic is that the defect deals with the loss on the smallest energy scale of light: that of the quantum
Quantum
In physics, a quantum is the minimum amount of any physical entity involved in an interaction. Behind this, one finds the fundamental notion that a physical property may be "quantized," referred to as "the hypothesis of quantization". This means that the magnitude can take on only certain discrete...
.
Quantum defect in laser scienceLaser scienceLaser science or laser physics is a branch of optics that describes the theory and practice of lasers.Laser science is principally concerned with quantum electronics, laser construction, optical cavity design, the physics of producing a population inversion in laser media, and the temporal...
In laserLaser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
science, the term quantum defect refers to the fact that the energy of a pump photon is generally higher than that of a signal photon (photon of the output radiation). The difference of energies goes to the heat;
this heat may carry away the excess of entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
delivered with the multimode uncoherent pump.
The quantum defect of a laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
can be defined as part of the energy of the pumping photon, which is lost (not turned into photons at the lasing wavelength) in the gain medium at the lasing.
At given frequency
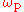 of pump
of pumpPump
A pump is a device used to move fluids, such as liquids, gases or slurries.A pump displaces a volume by physical or mechanical action. Pumps fall into three major groups: direct lift, displacement, and gravity pumps...
and given frequency
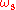 of lasing, the quantum defect
of lasing, the quantum defect 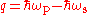 .
.Such quantum defect has dimension of energy; for the efficient operation, the temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
of the gain medium
(measured in units of energy) should be small compared to the quantum defect.
At a fixed pump frequency, the higher the quantum defect, the lower is the upper bound for the power efficiency.
Quantum defect in Rydberg atoms
The quantum defect of a Rydberg atomRydberg atom
thumb|right|300px|Figure 1: Energy levels in atomic [[lithium]] showing the Rydberg series of the lowest 3 values of [[Angular momentum#Angular momentum in quantum mechanics|orbital angular momentum]] converging on the first ionization energy....
refers to a correction applied to the equations governing Rydberg atom behavior to take into account the fact that the inner electrons do not entirely screen their associated charge in the nucleus. It is used particularity for the alkalis that contain a single electron in their outer shell.
The perfect 1/r potential in the hydrogen atom
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively-charged proton and a single negatively-charged electron bound to the nucleus by the Coulomb force...
leads to an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
binding energy
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
given by
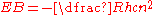 ,
,where R is the Rydberg constant
Rydberg constant
The Rydberg constant, symbol R∞, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to atomic spectra in the science of spectroscopy. Rydberg initially determined its value empirically from spectroscopy, but Niels Bohr later showed that its value could be calculated...
, h is Planck's constant, c is the speed of light and n is the principal quantum number
Principal quantum number
In atomic physics, the principal quantum symbolized as n is the firstof a set of quantum numbers of an atomic orbital. The principal quantum number can only have positive integer values...
.
For multi-electron atoms in Rydberg states with a low value of the orbital angular momentum, there is a high probability of finding the excited electron near the nucleus where it can polarize
Polarizability
Polarizability is the measure of the change in a molecule's electron distribution in response to an applied electric field, which can also be induced by electric interactions with solvents or ionic reagents. It is a property of matter...
or even penetrate the ion core, modifying the potential. The resulting shift of the energy levels is represented mathematically as an angular momentum dependent quantum defect, δl:
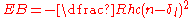 .
.The largest shifts occur when the orbital angular momentum is equal to 0 (normally labelled 's') and these are shown in the table for the alkali metals:
| Element | Configuration | n* | δs |
|---|---|---|---|
| Li | 2s | 1.59 | 0.41 |
| Na | 3s | 1.63 | 1.37 |
| K | 4s | 1.77 | 2.23 |
| Rb | 5s | 1.81 | 3.19 |
| Cs | 6s | 1.87 | 4.13 |

