
Protein precipitation
Encyclopedia
Precipitation is widely used in downstream processing
of biological products, such as proteins. http://www.rcsb.org/pdb/home/home.do This unit operation serves to concentrate and fractionate the target product from various contaminants. For example, in the biotechnology
industry protein precipitation is used to eliminate contaminants commonly contained in blood. http://www.pharmaceutical-int.com/categories/protein-precipitation/protein-precipitation-plates-tubes.asp Academic research on protein precipitation explores new protein precipitation methods. http://www.cchem.berkeley.edu/hwbgrp/research_files/protein_crystal.html The underlying mechanism of precipitation is to alter the solvation potential of the solvent and thus lower the solubility of the solute
by addition of a reagent.
of proteins in aqueous buffers depends on the distribution of hydrophilic and hydrophobic amino acid residues on the protein’s surface. Hydrophobic residues predominantly occur in the globular protein core, but some exist in patches on the surface. Proteins that have high hydrophobic amino acid
content on the surface have low solubility in an aqueous solvent. Charged and polar surface residues interact with ionic groups in the solvent and increase solubility. Knowledge of amino acid composition of a protein will aid in determining an ideal precipitation solvent and method.
solution. These repulsive forces between proteins prevent aggregation and facilitate dissolution. Solvent counterions migrate towards charged surface residues on the protein, forming a rigid matrix of counterions attached to the protein surface. The adjacent solvation layer, which is less rigid, consists of a decreasing concentration profile of the counterions and an increasing concentration profile of the co-ions. In effect, the protein’s potential to engage in ionic interactions with each other will decrease. Proteins will be less likely to form aggregates. Water molecules can have a similar effect. Water forms a solvation layer around hydrophilic surface residues of a protein. Water establishes a concentration gradient around the protein, with the highest concentration at the protein surface. This water network has a damping effect on the attractive forces between proteins.
phase, submicroscopic sized particles are generated. Growth of these particles is under Brownian diffusion control. Once the growing particles reach a critical size (0.1 µm to 10 µm for high and low shear fields, respectively), by diffusive addition of individual protein molecules, they continue to grow by colliding into each other and sticking or flocculating. This phase occurs at a slower rate. During the final step, aging in a shear field, the precipitate particles repeatedly collide and stick, then break apart, until a stable mean particle size is reached, which is dependent upon individual proteins. The mechanical strength of the protein particles correlates with the product of the mean shear rate and the aging time, which is known as the Camp number. Aging helps particles withstand the fluid shear forces encountered in pumps and centrifuge feed zones without reducing in size.
, compresses the solvation layer and increases protein-protein interactions. As the salt concentration of a solution is increased, more of the bulk water becomes associated with the ions. As a result, less water is available to partake in the solvation layer around the protein, which exposes hydrophobic patches on the protein surface. Proteins may then exhibit hydrophobic interactions, aggregate and precipitate from solution.
when the right concentration of the salt is reached in solution. The hydrophobic patches on the protein surface generate highly ordered water shells. This results in a small decrease in enthalpy
, ΔH, and a larger decrease in entropy
, ΔS, of the ordered water molecules relative to the molecules in the bulk solution. The overall free energy
change, ΔG, of the process is given by the Gibbs free energy equation: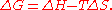
ΔG = Free energy change, ΔH = Enthalpy change upon precipitation, ΔS = Entropy change upon precipitation, T = Absolute temperature.
When water molecules in the rigid solvation layer are brought back into the bulk phase through interactions with the added salt, their greater freedom of movement causes a significant increase in their entropy. Thus, ΔG becomes negative and precipitation occurs spontaneously.
Hofmeister series
Kosmotropes or "water structure makers" are salts which promote the dissipation of water from the solvation layer around a protein. Hydrophobic patches are then exposed on the protein’s surface, and they interact with hydrophobic patches on other proteins. These salts enhance protein aggregation and precipitation. Chaotropes or “water structure breakers,” have the opposite effect of Kosmotropes. These salts promote an increase in the solvation layer around a protein. The effectiveness of the kosmotropic salts in precipitating proteins follows the order of the Hofmeister series:
Most precipitation least precipitation
least precipitation
Most precipitation least precipitation
least precipitation
equation:
S = solubility of the protein, B is idealized solubility, K is a salt-specific constant and I is the ionic strength of the solution, which is attributed to the added salt.
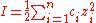
zi is the ion charge of the salt and ci is the salt concentration. The ideal salt for protein precipitation is most effective for a particular amino acid composition, inexpensive, non-buffering, and non-polluting. The most commonly used salt is ammonium sulfate
. There is a low variation in salting out over temperatures 0 °C to 30 °C. Protein precipitates left in the salt solution can remain stable for years-protected from proteolysis
and bacterial contamination by the high salt concentrations. Ammonium sulfate
salt cannot be used in solutions that have pH > 8 because the ammonium ion has a buffering effect on the solution. Sodium citrate
is a good alternative for solutions above pH 8.
(pI) is the pH of a solution at which the net primary charge of a protein becomes zero. At a solution pH that is above the pI the surface of the protein is predominantly negatively charged and therefore like-charged molecules will exhibit repulsive forces. Likewise, at a solution pH that is below the pI, the surface of the protein is predominantly positively charged and repulsion between proteins occurs. However, at the pI the negative and positive charges cancel, repulsive electrostatic forces are reduced and the attraction forces predominate. The attraction forces will cause aggregation and precipitation. The pI of most proteins is in the pH range of 4-6. Mineral acids, such as hydrochloric and sulfuric acid
are used as precipitants. The greatest disadvantage to isoelectric point precipitation is the irreversible denaturation
caused by the mineral acids. For this reason isoelectric point precipitation is most often used to precipitate contaminant proteins, rather than the target protein. The precipitation of casein during cheesemaking, or during production of sodium caseinate, is an isoelectric precipitation.
or methanol
to a solution may cause proteins in the solution to precipitate. The solvation layer around the protein will decrease as the organic solvent progressively displaces water from the protein surface and binds it in hydration layers around the organic solvent molecules. With smaller hydration layers, the proteins can aggregate by attractive electrostatic and dipole forces. Important parameters to consider are temperature, which should be less than 0 °C to avoid denaturation
, pH and protein concentration in solution. Miscible organic solvents decrease the dielectric constant
of water, which in effect allows two proteins to come close together. At the isoelectric point
the relationship between the dielectric constant and protein solubility is given by: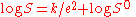
S0 is an extrapolated value of S, e is the dielectric constant of the mixture and k is a constant that relates to the dielectric constant of water. The Cohn process
for plasma protein fractionation relies on solvent precipitation with ethanol to isolate individual plasma proteins.
a clinical application for the use of methanol as a protein precipitating agent is in the estimation of bilirubin.
s, are frequently used to precipitate proteins because they have low flammability and are less likely to denature biomaterials than isoelectric precipitation. These polymers in solution attract water molecules away from the solvation layer around the protein. This increases the protein-protein interactions and enhances precipitation. For the specific case of polyethylene glycol, precipitation can be modeled by the equation: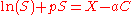
C is the polymer concentration, P is a protein-protein interaction coefficient, a is a protein-polymer interaction coefficient and
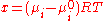
μ is the chemical potential
of component I, R is the universal gas constant and T is the absolute temperature.
and polyphosphates can form extended networks between protein molecules in solution. The effectiveness of these polyelectrolytes depend on the pH of the solution. Anionic polyelectrolytes are used at pH values less than the isoelectric point. Cationic polyelectrolytes are at pH values above the pI. It is important to note that an excess of polyelectrolytes will cause the precipitate to dissolve back into the solution. An example of polyelectrolyte flocculation is the removal of protein cloud from beer wort using Irish moss.
as they move though the tubes of the reactor. Turbulent flow is promoted through wire mesh inserts in the tube. The tubular reactor does not require moving mechanical parts and is inexpensive to build. However, the reactor can become impractically long if the particles aggregate slowly.
reactors run at steady state
with a continuous flow of reactants and products in a well-mixed tank. Fresh protein feed contacts slurry
that already contains precipitate particles and the precipitation reagents.
Downstream processing
Downstream processing refers to the recovery and purification of biosynthetic products, particularly pharmaceuticals, from natural sources such as animal or plant tissue or fermentation broth, including the recycling of salvageable components and the proper treatment and disposal of waste. It is an...
of biological products, such as proteins. http://www.rcsb.org/pdb/home/home.do This unit operation serves to concentrate and fractionate the target product from various contaminants. For example, in the biotechnology
Biotechnology
Biotechnology is a field of applied biology that involves the use of living organisms and bioprocesses in engineering, technology, medicine and other fields requiring bioproducts. Biotechnology also utilizes these products for manufacturing purpose...
industry protein precipitation is used to eliminate contaminants commonly contained in blood. http://www.pharmaceutical-int.com/categories/protein-precipitation/protein-precipitation-plates-tubes.asp Academic research on protein precipitation explores new protein precipitation methods. http://www.cchem.berkeley.edu/hwbgrp/research_files/protein_crystal.html The underlying mechanism of precipitation is to alter the solvation potential of the solvent and thus lower the solubility of the solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
by addition of a reagent.
Protein solubility
The solubilitySolubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
of proteins in aqueous buffers depends on the distribution of hydrophilic and hydrophobic amino acid residues on the protein’s surface. Hydrophobic residues predominantly occur in the globular protein core, but some exist in patches on the surface. Proteins that have high hydrophobic amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
content on the surface have low solubility in an aqueous solvent. Charged and polar surface residues interact with ionic groups in the solvent and increase solubility. Knowledge of amino acid composition of a protein will aid in determining an ideal precipitation solvent and method.
Repulsive electrostatic force
Repulsive electrostatic forces form when proteins are suspended in an electrolyteElectrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
solution. These repulsive forces between proteins prevent aggregation and facilitate dissolution. Solvent counterions migrate towards charged surface residues on the protein, forming a rigid matrix of counterions attached to the protein surface. The adjacent solvation layer, which is less rigid, consists of a decreasing concentration profile of the counterions and an increasing concentration profile of the co-ions. In effect, the protein’s potential to engage in ionic interactions with each other will decrease. Proteins will be less likely to form aggregates. Water molecules can have a similar effect. Water forms a solvation layer around hydrophilic surface residues of a protein. Water establishes a concentration gradient around the protein, with the highest concentration at the protein surface. This water network has a damping effect on the attractive forces between proteins.
Attractive electrostatic force
Dispersive or attractive forces exist between proteins through permanent and induced dipoles. For example, basic residues on a protein can have electrostatic interactions with acidic residues on another protein. However, solvation by ions in an electrolytic solution or water will decrease protein-protein attractive forces. Protein accumulation and precipitation can be enhanced by decreasing the hydration layer around the protein. The purpose of the added reagents in protein precipitation is to reduce the hydration layer.Precipitate formation
Protein precipitate formation occurs in a stepwise process. The addition of a precipitating agent and steady mixing destabilizes the protein solution. Mixing causes the precipitant and the target product to collide. Enough mixing time is required for molecules to diffuse across the fluid eddies. During the following nucleationNucleation
Nucleation is the extremely localized budding of a distinct thermodynamic phase. Some examples of phases that may form by way of nucleation in liquids are gaseous bubbles, crystals or glassy regions. Creation of liquid droplets in saturated vapor is also characterized by nucleation...
phase, submicroscopic sized particles are generated. Growth of these particles is under Brownian diffusion control. Once the growing particles reach a critical size (0.1 µm to 10 µm for high and low shear fields, respectively), by diffusive addition of individual protein molecules, they continue to grow by colliding into each other and sticking or flocculating. This phase occurs at a slower rate. During the final step, aging in a shear field, the precipitate particles repeatedly collide and stick, then break apart, until a stable mean particle size is reached, which is dependent upon individual proteins. The mechanical strength of the protein particles correlates with the product of the mean shear rate and the aging time, which is known as the Camp number. Aging helps particles withstand the fluid shear forces encountered in pumps and centrifuge feed zones without reducing in size.
Salting out
Salting out is the most common method used to precipitate a target protein. Addition of a neutral salt, such as ammonium sulfateAmmonium sulfate
Ammonium sulfate , 2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen as ammonium cations, and 24% sulfur as sulfate anions...
, compresses the solvation layer and increases protein-protein interactions. As the salt concentration of a solution is increased, more of the bulk water becomes associated with the ions. As a result, less water is available to partake in the solvation layer around the protein, which exposes hydrophobic patches on the protein surface. Proteins may then exhibit hydrophobic interactions, aggregate and precipitate from solution.
Energetics involved in salting out
Salting out is a spontaneous processSpontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
when the right concentration of the salt is reached in solution. The hydrophobic patches on the protein surface generate highly ordered water shells. This results in a small decrease in enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
, ΔH, and a larger decrease in entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
, ΔS, of the ordered water molecules relative to the molecules in the bulk solution. The overall free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
change, ΔG, of the process is given by the Gibbs free energy equation:
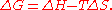
ΔG = Free energy change, ΔH = Enthalpy change upon precipitation, ΔS = Entropy change upon precipitation, T = Absolute temperature.
When water molecules in the rigid solvation layer are brought back into the bulk phase through interactions with the added salt, their greater freedom of movement causes a significant increase in their entropy. Thus, ΔG becomes negative and precipitation occurs spontaneously.
Hofmeister seriesHofmeister seriesThe Hofmeister series or lyotropic series is a classification of ions in order of their ability to salt out or salt in proteins. The effects of these changes were first worked out by Franz Hofmeister, who studied the effects of cations and anions on the solubility of proteins.Hofmeister discovered...
Kosmotropes or "water structure makers" are salts which promote the dissipation of water from the solvation layer around a protein. Hydrophobic patches are then exposed on the protein’s surface, and they interact with hydrophobic patches on other proteins. These salts enhance protein aggregation and precipitation. Chaotropes or “water structure breakers,” have the opposite effect of Kosmotropes. These salts promote an increase in the solvation layer around a protein. The effectiveness of the kosmotropic salts in precipitating proteins follows the order of the Hofmeister series:Most precipitation
 least precipitation
least precipitationMost precipitation
 least precipitation
least precipitationSalting out in practice
The decrease in protein solubility follows a normalized solubility curve of the type shown. The relationship between the solubility of a protein and increasing ionic strength of the solution can be represented by the CohnCohn
Cohn is a common Jewish surname and may refer to:* Al Cohn* Arthur Cohn, Swiss film producer* Daniel Cohn-Bendit * Dan Cohn-Sherbock* Edwin J Cohn **...
equation:

S = solubility of the protein, B is idealized solubility, K is a salt-specific constant and I is the ionic strength of the solution, which is attributed to the added salt.
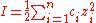
zi is the ion charge of the salt and ci is the salt concentration. The ideal salt for protein precipitation is most effective for a particular amino acid composition, inexpensive, non-buffering, and non-polluting. The most commonly used salt is ammonium sulfate
Ammonium sulfate
Ammonium sulfate , 2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen as ammonium cations, and 24% sulfur as sulfate anions...
. There is a low variation in salting out over temperatures 0 °C to 30 °C. Protein precipitates left in the salt solution can remain stable for years-protected from proteolysis
Proteolysis
Proteolysis is the directed degradation of proteins by cellular enzymes called proteases or by intramolecular digestion.-Purposes:Proteolysis is used by the cell for several purposes...
and bacterial contamination by the high salt concentrations. Ammonium sulfate
Ammonium sulfate
Ammonium sulfate , 2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen as ammonium cations, and 24% sulfur as sulfate anions...
salt cannot be used in solutions that have pH > 8 because the ammonium ion has a buffering effect on the solution. Sodium citrate
Sodium citrate
Trisodium citrate has the chemical formula of Na3C6H5O7. It is sometimes referred to simply as sodium citrate, though sodium citrate can refer to any of the three sodium salts of citric acid. It possesses a saline, mildly tart flavor. For this reason, citrates of certain alkaline and alkaline earth...
is a good alternative for solutions above pH 8.
Isoelectric point precipitation
The isoelectric pointIsoelectric point
The isoelectric point , sometimes abbreviated to IEP, is the pH at which a particular molecule or surface carries no net electrical charge....
(pI) is the pH of a solution at which the net primary charge of a protein becomes zero. At a solution pH that is above the pI the surface of the protein is predominantly negatively charged and therefore like-charged molecules will exhibit repulsive forces. Likewise, at a solution pH that is below the pI, the surface of the protein is predominantly positively charged and repulsion between proteins occurs. However, at the pI the negative and positive charges cancel, repulsive electrostatic forces are reduced and the attraction forces predominate. The attraction forces will cause aggregation and precipitation. The pI of most proteins is in the pH range of 4-6. Mineral acids, such as hydrochloric and sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
are used as precipitants. The greatest disadvantage to isoelectric point precipitation is the irreversible denaturation
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
caused by the mineral acids. For this reason isoelectric point precipitation is most often used to precipitate contaminant proteins, rather than the target protein. The precipitation of casein during cheesemaking, or during production of sodium caseinate, is an isoelectric precipitation.
Precipitation with organic solvents
Addition of miscible solvents such as ethanolEthanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
or methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
to a solution may cause proteins in the solution to precipitate. The solvation layer around the protein will decrease as the organic solvent progressively displaces water from the protein surface and binds it in hydration layers around the organic solvent molecules. With smaller hydration layers, the proteins can aggregate by attractive electrostatic and dipole forces. Important parameters to consider are temperature, which should be less than 0 °C to avoid denaturation
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
, pH and protein concentration in solution. Miscible organic solvents decrease the dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
of water, which in effect allows two proteins to come close together. At the isoelectric point
Isoelectric point
The isoelectric point , sometimes abbreviated to IEP, is the pH at which a particular molecule or surface carries no net electrical charge....
the relationship between the dielectric constant and protein solubility is given by:
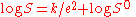
S0 is an extrapolated value of S, e is the dielectric constant of the mixture and k is a constant that relates to the dielectric constant of water. The Cohn process
Cohn process
The Cohn process is a series of purification steps with the purpose of extracting albumin from blood plasma. The process is based on the differential solubility of albumin and other plasma proteins based on pH, ethanol concentration, temperature, ionic strength, and protein concentration. Albumin...
for plasma protein fractionation relies on solvent precipitation with ethanol to isolate individual plasma proteins.
a clinical application for the use of methanol as a protein precipitating agent is in the estimation of bilirubin.
Non-ionic hydrophilic polymers
Polymers, such as dextrans and polyethylene glycolPolyethylene glycol
Polyethylene glycol is a polyether compound with many applications from industrial manufacturing to medicine. It has also been known as polyethylene oxide or polyoxyethylene , depending on its molecular weight, and under the tradename Carbowax.-Available forms:PEG, PEO, or POE refers to an...
s, are frequently used to precipitate proteins because they have low flammability and are less likely to denature biomaterials than isoelectric precipitation. These polymers in solution attract water molecules away from the solvation layer around the protein. This increases the protein-protein interactions and enhances precipitation. For the specific case of polyethylene glycol, precipitation can be modeled by the equation:
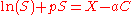
C is the polymer concentration, P is a protein-protein interaction coefficient, a is a protein-polymer interaction coefficient and
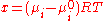
μ is the chemical potential
Chemical potential
Chemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
of component I, R is the universal gas constant and T is the absolute temperature.
Flocculation by polyelectrolytes
Alginate, carboxymethycellulose, polyacrylic acid, tannic acidTannic acid
Tannic acid is a specific commercial form of tannin, a type of polyphenol. Its weak acidity is due to the numerous phenol groups in the structure...
and polyphosphates can form extended networks between protein molecules in solution. The effectiveness of these polyelectrolytes depend on the pH of the solution. Anionic polyelectrolytes are used at pH values less than the isoelectric point. Cationic polyelectrolytes are at pH values above the pI. It is important to note that an excess of polyelectrolytes will cause the precipitate to dissolve back into the solution. An example of polyelectrolyte flocculation is the removal of protein cloud from beer wort using Irish moss.
Polyvalent metallic ions
Metal salts can be used at low concentrations to precipitate enzymes and nucleic acids from solutions. Polyvalent metal ions frequently used are Ca2+, Mg2+, Mn2+ or Fe2+.Precipitation reactors
There are numerous industrial scaled reactors than can be used to precipitate large amounts of proteins, such as recombinant DNA polymerases from a solution.http://cat.inist.fr/?aModele=afficheN&cpsidt=1394294Batch reactors
Batch reactors are the simplest type of precipitation reactor. The precipitating agent is slowly added to the protein solution under mixing. The aggregating protein particles tend to be compact and regular in shape. Since the particles are exposed to a wide range of shear stresses for a long period of time, they tend to be compact, dense and mechanically stable.Tubular reactors
In tubular reactors, feed protein solution and the precipitating reagent are contacted in a zone of efficient mixing then fed into long tubes where precipitation takes place. The fluid in volume elements approach plug flowPlug flow
In fluid mechanics, plug flow is a simple model of the velocity profile of a fluid flowing in a pipe. In plug flow, the velocity of the fluid is assumed to be constant across any cross-section of the pipe perpendicular to the axis of the pipe...
as they move though the tubes of the reactor. Turbulent flow is promoted through wire mesh inserts in the tube. The tubular reactor does not require moving mechanical parts and is inexpensive to build. However, the reactor can become impractically long if the particles aggregate slowly.
Continuous stirred tank reactors (CSTR)
CSTRContinuous stirred-tank reactor model
The continuous stirred-tank reactor , also known as vat- or backmix reactor, is a common ideal reactor type in chemical engineering. A CSTR often refers to a model used to estimate the key unit operation variables when using a continuous agitated-tank reactor to reach a specified output...
reactors run at steady state
Steady state
A system in a steady state has numerous properties that are unchanging in time. This implies that for any property p of the system, the partial derivative with respect to time is zero:...
with a continuous flow of reactants and products in a well-mixed tank. Fresh protein feed contacts slurry
Slurry
A slurry is, in general, a thick suspension of solids in a liquid.-Examples of slurries:Examples of slurries include:* Lahars* A mixture of water and cement to form concrete* A mixture of water, gelling agent, and oxidizers used as an explosive...
that already contains precipitate particles and the precipitation reagents.

