
Continuous stirred-tank reactor model
Encyclopedia
The continuous stirred-tank reactor (CSTR), also known as vat- or backmix reactor, is a common ideal reactor type in chemical engineering
. A CSTR often refers to a model used to estimate the key unit operation variables when using a continuous agitated-tank reactor to reach a specified output. (See Chemical reactor
s.) The mathematical model works for all fluids: liquids, gases, and slurries
.
The behavior of a CSTR is often approximated or modeled by that of a Continuous Ideally Stirred-Tank Reactor (CISTR). All calculations performed with CISTRs assume perfect mixing
. In a perfectly mixed reactor, the output composition is identical to composition of the material inside the reactor, which is a function of residence time and rate of reaction. If the residence time is 5-10 times the mixing time, this approximation is valid for engineering purposes. The CISTR model is often used to simplify engineering calculations and can be used to describe research reactors. In practice it can only be approached, in particular in industrial size reactors.
Assume:
Integral mass balance on number of moles Ni of species i in a reactor of volume V.
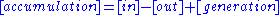
1.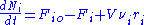
where Fio is the molar flow rate inlet of species i, Fi the molar flow rate outlet, and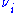 stoichiometric coefficient
stoichiometric coefficient
. The reaction rate, r, is generally dependent on the reactant concentration and the rate constant (k). The rate constant can be determined by using a known empirical reaction rates that is adjusted for temperature using the Arrhenius temperature dependence
. Generally, as the temperature increases so does the rate at which the reaction occurs. Residence time
, , is the average amount of time a discrete quantity of reagent spends inside the tank.
, is the average amount of time a discrete quantity of reagent spends inside the tank.
Assume:
A → products
NA = CA V (where CA is the concentration of species A, V is the volume of the reactor, NA is the number of moles of species A)
2.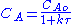
The values of the variables, outlet concentration and residence time, in Equation 2 are major design criteria.
To model systems that do not obey the assumptions of constant temperature and a single reaction, additional dependent variables must be considered. If the system is considered to be in unsteady-state, a differential equation or a system of coupled differential equations must be solved.
CSTR's are known to be one of the systems which exhibit complex behavior such as steady-state multiplicity, limit cycles and chaos.
3. Non-ideal CSTR
The actual reaction volume will be affected by the non-uniform mixing (short-circuiting) or existence of dead zone areas. Temperature effect will also cause significant effect on the reaction rate.
Chemical engineering
Chemical engineering is the branch of engineering that deals with physical science , and life sciences with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms...
. A CSTR often refers to a model used to estimate the key unit operation variables when using a continuous agitated-tank reactor to reach a specified output. (See Chemical reactor
Chemical reactor
In chemical engineering, chemical reactors are vessels designed to contain chemical reactions. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction...
s.) The mathematical model works for all fluids: liquids, gases, and slurries
Slurry
A slurry is, in general, a thick suspension of solids in a liquid.-Examples of slurries:Examples of slurries include:* Lahars* A mixture of water and cement to form concrete* A mixture of water, gelling agent, and oxidizers used as an explosive...
.
The behavior of a CSTR is often approximated or modeled by that of a Continuous Ideally Stirred-Tank Reactor (CISTR). All calculations performed with CISTRs assume perfect mixing
Perfect mixing
Perfect mixing is a term heavily used in relation to the definition of models that predict the behavior of chemical reactors. Perfect mixing assumes that there are no spatial gradients in a given physical envelope, such as:...
. In a perfectly mixed reactor, the output composition is identical to composition of the material inside the reactor, which is a function of residence time and rate of reaction. If the residence time is 5-10 times the mixing time, this approximation is valid for engineering purposes. The CISTR model is often used to simplify engineering calculations and can be used to describe research reactors. In practice it can only be approached, in particular in industrial size reactors.
Assume:
- perfect or ideal mixing, as stated above
Integral mass balance on number of moles Ni of species i in a reactor of volume V.
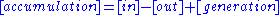
1.
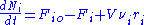
where Fio is the molar flow rate inlet of species i, Fi the molar flow rate outlet, and
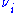 stoichiometric coefficient
stoichiometric coefficientStoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
. The reaction rate, r, is generally dependent on the reactant concentration and the rate constant (k). The rate constant can be determined by using a known empirical reaction rates that is adjusted for temperature using the Arrhenius temperature dependence
Arrhenius equation
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
. Generally, as the temperature increases so does the rate at which the reaction occurs. Residence time
Residence time
Residence time is the average amount of time that a particle spends in a particular system. This measurement varies directly with the amount of substance that is present in the system....
,
 , is the average amount of time a discrete quantity of reagent spends inside the tank.
, is the average amount of time a discrete quantity of reagent spends inside the tank.Assume:
- constant densityDensityThe mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
(valid for most liquids; valid for gases only if there is no net change in the number of moles or drastic temperature change) - isothermal conditions, or constant temperature (k is constant)
- steady stateSteady-state (chemical engineering)A unit operation is considered to be at a steady state with respect to an operation variable if that variable does not change with time. Such a process is called a steady-state process.-Example:...
- single, irreversible reactionChemical reactionA chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
(νA = -1) - first-order reaction (r = kCA)
A → products
NA = CA V (where CA is the concentration of species A, V is the volume of the reactor, NA is the number of moles of species A)
2.
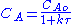
The values of the variables, outlet concentration and residence time, in Equation 2 are major design criteria.
To model systems that do not obey the assumptions of constant temperature and a single reaction, additional dependent variables must be considered. If the system is considered to be in unsteady-state, a differential equation or a system of coupled differential equations must be solved.
CSTR's are known to be one of the systems which exhibit complex behavior such as steady-state multiplicity, limit cycles and chaos.
3. Non-ideal CSTR
The actual reaction volume will be affected by the non-uniform mixing (short-circuiting) or existence of dead zone areas. Temperature effect will also cause significant effect on the reaction rate.

