
Prefoldin
Encyclopedia
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s used in protein folding
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
complexes. It is classified as a heterohexameric molecular chaperone in both archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
and eukarya, including human
Human
Humans are the only living species in the Homo genus...
s. A prefoldin molecule works as a transfer protein in conjunction with a molecule of chaperonin
Chaperonin
Chaperonins are proteins that fold and unfold other proteins. Newly made proteins usually must fold from a linear chain of amino acids into a three-dimensional form. Chaperonins belong to a large class of molecules that assist protein folding, called molecular chaperones...
to form a chaperone complex and correctly fold other nascent proteins. One of prefoldin's main uses in eukarya is the formation of molecules of actin
Actin
Actin is a globular, roughly 42-kDa moonlighting protein found in all eukaryotic cells where it may be present at concentrations of over 100 μM. It is also one of the most highly-conserved proteins, differing by no more than 20% in species as diverse as algae and humans...
for use in the eukaryotic cytoskeleton
Cytoskeleton
The cytoskeleton is a cellular "scaffolding" or "skeleton" contained within a cell's cytoplasm and is made out of protein. The cytoskeleton is present in all cells; it was once thought to be unique to eukaryotes, but recent research has identified the prokaryotic cytoskeleton...
.
Purpose and Uses
Prefoldin is one family of chaperone proteins found in the domains of eukarya and archaea. Prefoldin acts in combination with other molecules as a chaperone protein and promotes protein folding in cells where there are many other competing pathways for protein folding . Chaperone proteins perform non-covalent assembly of other polypeptide-containing structures in vivo. They are implicated in the folding of most other proteins. In archaea, prefoldins are believed to function in combination with group II chaperonins in de novo protein foldingProtein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
, similar to other chaperone complexes. In eukarya however, prefoldins have acquired a more specific function; they are used to establish correct tubular assembly for many tubular proteins, such as actin
Actin
Actin is a globular, roughly 42-kDa moonlighting protein found in all eukaryotic cells where it may be present at concentrations of over 100 μM. It is also one of the most highly-conserved proteins, differing by no more than 20% in species as diverse as algae and humans...
. Actin accounts for 5-10% of all protein found in eukaryotic cells, which therefore means that prefoldin is quite prevalent in the cells. Actin is made of two strings of beads wound round each other and is one of the three main parts of the cytoskeleton
Cytoskeleton
The cytoskeleton is a cellular "scaffolding" or "skeleton" contained within a cell's cytoplasm and is made out of protein. The cytoskeleton is present in all cells; it was once thought to be unique to eukaryotes, but recent research has identified the prokaryotic cytoskeleton...
of eukaryotic cells . The prefoldin bonds specifically to cytosolic chaperonin protein in the cytosol. This complex of prefoldin and chaperonin then forms molecules of actin in the cytosol of eukaryotic cells. The prefoldin acts as a transporter molecule that transports bound unfolded target proteins to the chaperonin (C-CPN) molecule . For example, the prefoldin that is used in the formation of actin transfers β tubulin or α tubulin to a cytosolic chaperonin. The prefoldin, however, does not form a ternary complex with either tubulin and chaperonin. Once the tubulins are in contact with the chaperonin, the prefoldin automatically lets go and leaves the active site. This is due for prefoldin’s high affinity for the cytosolic chaperonin molecule, as once the prefoldin is in contact with the chaperonin protein, it loses its affinity for the unfolded target protein. Prefoldin is triggered only to bind to nonnative target proteins in the cytosol so that it will only bind to unfolded proteins. Unlike many other molecular chaperones, prefoldin does not use chemical energy, in the form of Adenosine triphosphate
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(ATP), to promote protein folding in all cells .
Discovery
Prefoldin was found by the laboratory of Nicholas J. Cowan from the Department of Biochemistry at the New York UniversityNew York University
New York University is a private, nonsectarian research university based in New York City. NYU's main campus is situated in the Greenwich Village section of Manhattan...
Medical Center. It was discovered using chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
. Unfolded labeled β-actin from bovine testes was put into solution. This solution contained an excess of cytosolic chaperonin (C-CPN), a eukaryotic chaperone protein necessary for actin folding. After gel filtration of the actin, the actin complex, consisting of actin and its bonded proteins, began to form and the molecular weight of the complex was observed. Gel electrophoresis was used to analyze the protein complex, the complex formed a single band that was excised and ran on an SDS gel. It resolved into five bands, therefore proving that a heterooligomeric protein is used to bind to unfolded actin . The discovery paper was published on May 29, 1998 in the journal Cell. The laboratory of Ulrich Hartl at the Max Planck Institute for Biochemistry in Martinsried, Germany, has identified an archaeal homolog of prefoldin that also functions as a molecular chaperone . Eukaryotic prefoldin likely evolved from archaea, as it is not present (or has been lost) from bacteria.
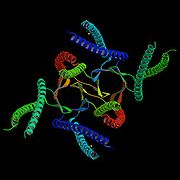
Structure
Prefoldin is a hetero hexameric protein consisting of two α subunits and four β subunits. The structure of the archaeal homologue was first solved by Siegert et al.. . Another archaeal prefoldin structure was then published by Clark et al.. . The beta subunits contain 120 amino acidAmino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues each, while the α subunits contain 140 amino acid residues each . Each subunit was found to have a width of 8.4 nm in the archaea Methanococcus thermoautrophicum . The height was calculated at 1.8-2.6 nm . The subunits are arranged by hydrophobic interactions with two β barrels at the center and coiled-coil α helices protruding down from them as if it were a jellyfish
Jellyfish
Jellyfish are free-swimming members of the phylum Cnidaria. Medusa is another word for jellyfish, and refers to any free-swimming jellyfish stages in the phylum Cnidaria...
.
See also
- Chaperone
- ActinActinActin is a globular, roughly 42-kDa moonlighting protein found in all eukaryotic cells where it may be present at concentrations of over 100 μM. It is also one of the most highly-conserved proteins, differing by no more than 20% in species as diverse as algae and humans...
- ChaperoninChaperoninChaperonins are proteins that fold and unfold other proteins. Newly made proteins usually must fold from a linear chain of amino acids into a three-dimensional form. Chaperonins belong to a large class of molecules that assist protein folding, called molecular chaperones...
- Protein FoldingProtein foldingProtein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....

