
Phosphoribosylanthranilate isomerase
Encyclopedia
In enzymology, a phosphoribosylanthranilate isomerase is an enzyme
that catalyzes
the third step of tryptophan biosynthesis.
Hence, this enzyme has one substrate
, N-(5-phospho-beta-D-ribosyl)anthranilate, and one product
, 1-(2-carboxyphenylamino)-1-deoxy-D-ribulose 5-phosphate.
This enzyme belongs to the family of isomerase
s, specifically those intramolecular oxidoreductase
s interconverting aldose
s and ketose
s. The systematic name of this enzyme class is N-(5-phospho-beta-D-ribosyl)anthranilate aldose-ketose-isomerase. Other names in common use include PRA isomerase, PRAI, IGPS:PRAI (indole-3-glycerol-phosphate, synthetase/N-5'-phosphoribosylanthranilate isomerase complex), and N-(5-phospho-beta-D-ribosyl)anthranilate ketol-isomerase. This enzyme participates in phenylalanine
, tyrosine
and tryptophan
biosynthesis
.
and stable in the hyperthermophile Thermotoga maritima (tPRAI). The comparison to the known 2.0 A structure
of PRAI from Escherichia coli (ePRAI) shows that tPRAI has a TIM-barrel
fold, whereas helix alpha5 in ePRAI is replaced by a loop
. The subunits
of tPRAI associate via the N-terminal faces of their central beta-barrels. Two long, symmetry-related loops that protrude reciprocally into cavities of the other subunit provide for multiple hydrophobic interactions. Moreover, the side chains of the N-terminal methionine
s and the C-terminal leucines
of both subunits
are immobilized in a hydrophobic
cluster, and the number of salt
bridges is increased in tPRAI. These features appear to be mainly responsible for the high thermostability of tPRAI.
As of late 2007, 5 structures
have been solved for this class of enzymes, with PDB
accession codes , , , , and .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the third step of tryptophan biosynthesis.
- N-(5-phospho-beta-D-ribosyl)anthranilate
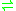 1-(2-carboxyphenylamino)-1-deoxy-D-ribulose 5-phosphate
1-(2-carboxyphenylamino)-1-deoxy-D-ribulose 5-phosphate
Hence, this enzyme has one substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
, N-(5-phospho-beta-D-ribosyl)anthranilate, and one product
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
, 1-(2-carboxyphenylamino)-1-deoxy-D-ribulose 5-phosphate.
This enzyme belongs to the family of isomerase
Isomerase
In biochemistry, an isomerase is an enzyme that catalyzes the structural rearrangement of isomers. Isomerases thus catalyze reactions of the formwhere B is an isomer of A.-Nomenclature:...
s, specifically those intramolecular oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s interconverting aldose
Aldose
An aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
s and ketose
Ketose
A ketose is a sugar containing one ketone group per molecule.With 3 carbon atoms, dihydroxyacetone is the simplest of all ketoses and is the only one having no optical activity. Ketoses can isomerize into an aldose when the carbonyl group is located at the end of the molecule...
s. The systematic name of this enzyme class is N-(5-phospho-beta-D-ribosyl)anthranilate aldose-ketose-isomerase. Other names in common use include PRA isomerase, PRAI, IGPS:PRAI (indole-3-glycerol-phosphate, synthetase/N-5'-phosphoribosylanthranilate isomerase complex), and N-(5-phospho-beta-D-ribosyl)anthranilate ketol-isomerase. This enzyme participates in phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
, tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
and tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
biosynthesis
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
.
Structural studies
Phosphoribosylanthranilate isomerase (PRAI) is monomeric and labile in most mesophilic microorganisms, but dimericProtein dimer
In biochemistry, a dimer is a macromolecular complex formed by two, usually non-covalently bound, macromolecules like proteins or nucleic acids...
and stable in the hyperthermophile Thermotoga maritima (tPRAI). The comparison to the known 2.0 A structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
of PRAI from Escherichia coli (ePRAI) shows that tPRAI has a TIM-barrel
TIM barrel
The TIM barrel is a conserved protein fold consisting of eight α-helices and eight parallel β-strands that alternate along the peptide backbone. The structure is named after triosephosphate isomerase, a conserved glycolytic enzyme. TIM barrels are quite common among the conserved protein folds...
fold, whereas helix alpha5 in ePRAI is replaced by a loop
Turn (biochemistry)
A turn is an element of secondary structure in proteins where the polypeptide chain reverses its overall direction.- Definition :According to the most common definition, a turn is a structural motif where the Cα atoms of two residues separated by few peptide bonds are in close approach A turn is...
. The subunits
Protein subunit
In structural biology, a protein subunit or subunit protein is a single protein molecule that assembles with other protein molecules to form a protein complex: a multimeric or oligomeric protein. Many naturally occurring proteins and enzymes are multimeric...
of tPRAI associate via the N-terminal faces of their central beta-barrels. Two long, symmetry-related loops that protrude reciprocally into cavities of the other subunit provide for multiple hydrophobic interactions. Moreover, the side chains of the N-terminal methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
s and the C-terminal leucines
Leucines
The leucines are primarily the four isomeric amino acids: leucine, isoleucine, tert-leucine and norleucine. Being compared with the four butanols, they could be classified as butyl-substituted glycines; they represent all four possible variations....
of both subunits
Protein subunit
In structural biology, a protein subunit or subunit protein is a single protein molecule that assembles with other protein molecules to form a protein complex: a multimeric or oligomeric protein. Many naturally occurring proteins and enzymes are multimeric...
are immobilized in a hydrophobic
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
cluster, and the number of salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
bridges is increased in tPRAI. These features appear to be mainly responsible for the high thermostability of tPRAI.
As of late 2007, 5 structures
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , , and .

