
Phenylalanine-tRNA ligase
Encyclopedia
In enzymology, a phenylalanine-tRNA ligase is an enzyme
that catalyzes
the chemical reaction
The 3 substrates
of this enzyme are ATP
, L-phenylalanine, and tRNA(Phe), whereas its 3 products
are AMP
, diphosphate, and L-phenylalanyl-tRNA(Phe).
This enzyme belongs to the family of ligase
s, to be specific those forming carbon-oxygen bonds in aminoacyl-tRNA and related compounds. The systematic name of this enzyme class is L-phenylalanine:tRNAPhe ligase (AMP-forming). Other names in common use include phenylalanyl-tRNA synthetase, phenylalanyl-transfer ribonucleate synthetase, phenylalanine-tRNA synthetase, phenylalanyl-transfer RNA synthetase, phenylalanyl-tRNA ligase, phenylalanyl-transfer RNA ligase, L-phenylalanyl-tRNA synthetase, and phenylalanine translase. This enzyme participates in phenylalanine, tyrosine and tryptophan biosynthesis and aminoacyl-trna biosynthesis.
Phenylalanine-tRNA synthetase (PheRS) is known to be among the most complex enzyme
s of the aaRS (Aminoacyl-tRNA synthetase) family. Bacterial
and mitochondrial
PheRSs share a ferredoxin
-fold anticodon binding (FDX-ACB) domain
, which represents a canonical double split alpha+beta motif having no insertions. The FDX-ACB domain displays a typical RNA recognition fold
(RRM) formed by the four-stranded antiparallel
beta sheet, with two helices
packed against it.
have been solved for this class of enzymes, with PDB
accession codes , , , , , , , , , and .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- ATP + L-phenylalanine + tRNAPhe
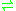 AMP + diphosphate + L-phenylalanyl-tRNAPhe
AMP + diphosphate + L-phenylalanyl-tRNAPhe
The 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, L-phenylalanine, and tRNA(Phe), whereas its 3 products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are AMP
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
, diphosphate, and L-phenylalanyl-tRNA(Phe).
This enzyme belongs to the family of ligase
Ligase
In biochemistry, ligase is an enzyme that can catalyse the joining of two large molecules by forming a new chemical bond, usually with accompanying hydrolysis of a small chemical group dependent to one of the larger molecules...
s, to be specific those forming carbon-oxygen bonds in aminoacyl-tRNA and related compounds. The systematic name of this enzyme class is L-phenylalanine:tRNAPhe ligase (AMP-forming). Other names in common use include phenylalanyl-tRNA synthetase, phenylalanyl-transfer ribonucleate synthetase, phenylalanine-tRNA synthetase, phenylalanyl-transfer RNA synthetase, phenylalanyl-tRNA ligase, phenylalanyl-transfer RNA ligase, L-phenylalanyl-tRNA synthetase, and phenylalanine translase. This enzyme participates in phenylalanine, tyrosine and tryptophan biosynthesis and aminoacyl-trna biosynthesis.
Phenylalanine-tRNA synthetase (PheRS) is known to be among the most complex enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s of the aaRS (Aminoacyl-tRNA synthetase) family. Bacterial
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and mitochondrial
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
PheRSs share a ferredoxin
Ferredoxin
Ferredoxins are iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co...
-fold anticodon binding (FDX-ACB) domain
Protein domain
A protein domain is a part of protein sequence and structure that can evolve, function, and exist independently of the rest of the protein chain. Each domain forms a compact three-dimensional structure and often can be independently stable and folded. Many proteins consist of several structural...
, which represents a canonical double split alpha+beta motif having no insertions. The FDX-ACB domain displays a typical RNA recognition fold
RNA recognition motif
RNA recognition motif, RNP-1 is a putative RNA-binding domain of about 90 amino acids that are known to bind single-stranded RNAs. It was found in many eukaryotic proteins....
(RRM) formed by the four-stranded antiparallel
Antiparallel (biochemistry)
In biochemistry, two molecules are antiparallel if they run side-by-side in opposite directions or when both strands are complimentary to each other....
beta sheet, with two helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
packed against it.
Structural studies
As of late 2007, 10 structuresTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , , , , , , , , and .

