
Peroxodisulfate
Encyclopedia
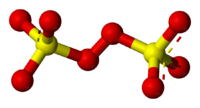
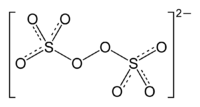
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
oxoanion
Oxyanion
An oxyanion or oxoanion is a chemical compound with the generic formula AxOyz− . Oxoanions are formed by a large majority of the chemical elements. The formulae of simple oxoanions are determined by the octet rule...
. It is commonly referred to as the persulfate
Persulfate
The term persulfate refers to ions or compounds with more oxygen than normal sulfates.These do not have sulfur in a different oxidation state; rather, they contain peroxide units, where two oxygens take the place of one in a normal sulfate; the oxygen atoms are in oxidation state −1.The main forms...
ion, but this term also refers to the peroxomonosulfate
Peroxomonosulfate
The peroxomonosulfate ion, SO52−, is a sulfur oxoanion. It is sometimes referred to as the persulfate ion, but this term also refers to the peroxodisulfate ion, S2O82−....
ion, SO52−.
Compounds containing peroxodisulfate
- Na2S2O8Sodium persulfateSodium persulfate is a chemical compound. It is a strong oxidizer. It is a severe irritant of skin, eyes, and respiratory system. It is almost non-hygroscopic and has particularly good ability to be stored for long time. It is easy and safe to handle...
- K2S2O8
- (NH4)2S2O8Ammonium persulfateAmmonium persulfate 2S2O8 is a strong oxidizing agent. It is very soluble in water; the dissolution of the salt in water is endothermic. It is a radical initiator. It is used to etch copper on printed circuit boards as an alternative to ferric chloride solution...
Reactions that use peroxodisulfate
- Elbs persulfate oxidationElbs persulfate oxidationThe Elbs persulfate oxidation is the organic reaction of phenols with alkaline potassium persulfate to form para-diphenols.Several reviews have been published.-Reaction mechanism:...
- Oxidation of Ag+ to Ag2+

