
Penny battery
Encyclopedia
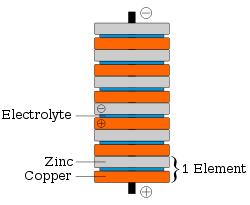
Voltaic pile
A voltaic pile is a set of individual Galvanic cells placed in series. The voltaic pile, invented by Alessandro Volta in 1800, was the first electric battery...
which uses various coinage
Coin
A coin is a piece of hard material that is standardized in weight, is produced in large quantities in order to facilitate trade, and primarily can be used as a legal tender token for commerce in the designated country, region, or territory....
as the metal disks of a traditional voltaic pile. The coins are stacked with pieces of electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
soaked paper in between (see diagram at right). The penny battery experiment is often during electrochemistry
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
units in an educational setting.
Each cell
Electrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
in a penny battery can produce up to 0.8 volts, and many can be stacked together to produce higher voltages. Since the battery is a wet cell, the effectiveness will be reduced when the electrolyte evaporates.
Coinage selection
As the name implies, Canadian penniesPenny (Canadian coin)
In Canada, a penny is a coin worth one cent or of a dollar. According to the Royal Canadian Mint, the official national term of the coin is the "one-cent piece", but in practice the term penny or cent is universal. Originally, "penny" referred to a two-cent coin. When the two-cent coin was...
from 1997-1999 may serve the zinc electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
and 1942-1996 pennies as the copper. Alternatively, American pennies
Cent (United States coin)
The United States one-cent coin, commonly known as a penny, is a unit of currency equaling one one-hundredth of a United States dollar. The cent's symbol is ¢. Its obverse has featured the profile of President Abraham Lincoln since 1909, the centennial of his birth. From 1959 to 2008, the reverse...
from 1982-present may be used as the zinc electrodes and 1944-1982 pennies as the copper electrodes. A variety of other coins may also be used, with varying results.

